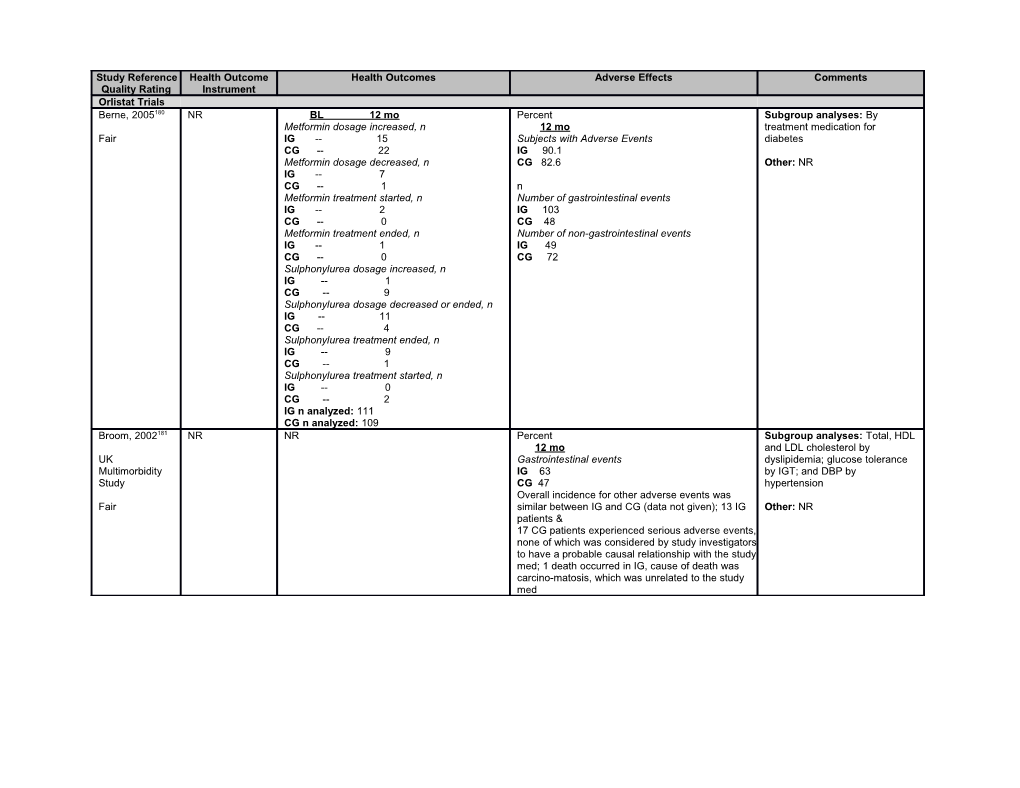
Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument Orlistat Trials Berne, 2005180 NR BL 12 mo Percent Subgroup analyses: By Metformin dosage increased, n 12 mo treatment medication for Fair IG -- 15 Subjects with Adverse Events diabetes CG -- 22 IG 90.1 Metformin dosage decreased, n CG 82.6 Other: NR IG -- 7 CG -- 1 n Metformin treatment started, n Number of gastrointestinal events IG -- 2 IG 103 CG -- 0 CG 48 Metformin treatment ended, n Number of non-gastrointestinal events IG -- 1 IG 49 CG -- 0 CG 72 Sulphonylurea dosage increased, n IG -- 1 CG -- 9 Sulphonylurea dosage decreased or ended, n IG -- 11 CG -- 4 Sulphonylurea treatment ended, n IG -- 9 CG -- 1 Sulphonylurea treatment started, n IG -- 0 CG -- 2 IG n analyzed: 111 CG n analyzed: 109 Broom, 2002181 NR NR Percent Subgroup analyses: Total, HDL 12 mo and LDL cholesterol by UK Gastrointestinal events dyslipidemia; glucose tolerance Multimorbidity IG 63 by IGT; and DBP by Study CG 47 hypertension Overall incidence for other adverse events was Fair similar between IG and CG (data not given); 13 IG Other: NR patients & 17 CG patients experienced serious adverse events, none of which was considered by study investigators to have a probable causal relationship with the study med; 1 death occurred in IG, cause of death was carcino-matosis, which was unrelated to the study med Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument Davidson, NR NR Percent Subgroup analyses: NR 1999182 12 mo Withdrawn because of adverse events Other: Subjects in IG were Fair IG 9.1 (calc) rerandomized after 12 months. CG 4.0 (calc) This data is not abstracted due to At least 1 gastrointestinal event the high loss of participants after IG 79 that point CG 59 Vitamin deficiency: Vitamin D and E levels decreased significantly in IG but mean levels within reference range; 14% IG need vitamin supplementation compared to 6.5% CG over 2 years Derosa, 2003183 NR NR Percent Subgroup analyses: NR 12 mo Fair Participants dropping out due to adverse events, Other: NR percent IG 7.4 CG 0
No serious adverse events. Derosa, 2010215 NR NR n (percent) Subgroup analyses: NR 12 mo Good Withdrew due to adverse events Other: NR IG 13 (10.3) CG 4 (3.1)
Majority of reasons for withdrawal (92.3%) were GI related Other AEs: Flatulence, constipation, fatty/oily evacuation, increased defecation, fecal urgency, malaise Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument Finer, 2000184 NR NR 12 mo Subgroup analyses: NR Withdrew because of adverse events, percent James, 1997290 IG 8.0 Other: NR CG 6.4 Fair At least one gastrointestinal event, percent IG 82.1 CG 56.4
Other AEs: Loose stools, Increased defecation, Abdominal pain, Uncontrolled oily discharge, Fecal urgency, Nausea/vomiting, Discolored feces, Flatulence, Decreased defecation, Upper respiratory tract infection, Pharyngitis, Influenza/influenza syndrome, Headache, Back pain, Gallbladder abnormalities, Renal abnormalities, Mild severity AE*, Moderate severity AE*, Unrelated to test drug AE*, Remotely related to test drug AE*, Possibly related to test drug AE*, Probably related to test drug*, List of AE leading to withdrawl in IG and CG*
N (percent) 12 mo Patients with adverse events* IG 23 (100) CG 21 (91.3) Severe Severity* IG 3 (13) CG 6 (26)
* From a subsample of patients only seen at the Aberdeen center (n=23 in IG and n=23 in CG) Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument Hanefeld, NR Mean change from BL at 12 mo 12 mo Subgroup analyses: Patients 2002187 -4 week 12 mo At least one adverse event, percent with type 2 diabetes previously Anti-DM medication dosage decreased or IG 89 treated with diet alone; effects of Fair ended, percent CG 88 IG in patients not on DM IG -- 9.7 At least one gastrointestinal event, percent medication at baseline CG -- 9.0 IG 76 Anti-DM medication dosage increased or CG 46 Other: NR started, percent Severe gastrointestinal event, n IG -- 14.0 IG 6 CG -- 17.5 CG 5 Withdrew because of GI events related to mode of action of orlistat, percent IG 4 CG 2 Hypoglycemia (at least 1 episode), n IG 2 CG 4 All hypoglycemic episodes were mild or moderate and none resulted in hospitalization or any adjustment in antidiabetic medication. No apparent differences in clinical laboratory parameters or vital signs between treatment groups were noted. Levels of fat-soluble vitamins were generally lower in IG than CG, but remained in normal ranges. Hauptman, NR N (percent) 24 mo Subgroup analyses: NR 2000189 24 mo Withdrew because of adverse event, percent Died (acute myocardial infarction) IG1 6.6 Other: 24 month data not Fair IG1 0 (0) IG2 11.0 abstracted because of high IG2 1 (0.5) CG 7.1 attrition CG 0 (0) Withdrew because of GI adverse event, percent IG1 4.7 IG2 5.7 CG 1.4 GI events, percent IG1 72** IG2 79** CG 59 Requiring supplementation with β-carotene, percent IG1 4.3 IG2 6.3 CG 2.4 Other AEs: Fecal urgency*, oily spotting*, fatty/oily stool*, flatus with discharge*, oOily evacuation*, increased defecation*, fecal incontinence*, 2+ consecutive low vitamin levels for vitamin A, E*, D and β-carotene** * p<0.005 for IG versus CG ** p<0.01 for IG versus CG <1.9% of all patients required and received vitamin A or E supplementation. Almost all patients who needed vitamin supplementation achieved normal Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument levels by the end of the study.
(continued) Most GI events were mild-moderate in intensity, Hauptman, limited to 1-2 episodes/patient, and occurred early 2000189 in treatment. AEs in all groups were transient, mild, or moderate in intensity and resolved without Fair intervention. With the exception of GI events, incidence and type of adverse events were similar in all treatment groups. Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument Hill, 1999190 NR NR % of subjects who reported ≥1 AEs was ~7-8% Subgroup analyses: NR greater in IG than CG Fair 12 mo Other: NR Reporting gastrointestinal events, percent IG1 82.3 IG2 91.8 IG3 95.0 CG 68.1 Withdrawals related to gastrointestinal events, percent IG1 5.4 IG2 7.0 IG3 11.7 CG 0.5 Other AEs: Flatus with discharge, abdominal pain, fecal urgency, oily spotting. Most subjects experienced only 1-2 episodes and most GI events were mild-moderate in intensity, occurred early during treatment, and resolved spontaneously. Vitamin E and β-carotene were significantly lower in IGs compared to CG at end of study (p<0.001). <4% of subjects met criteria for additional vitamin supplementation and those who did had normal values at end of study. Hollander, NR Percent change 12 mo Subgroup analyses: HbA1c 1998191 Percent change in average dose of oral % with ≥1 GI event presented for those with levels of sulfonylurea medication IG 79 >8% at BL; cholesterol, LDL, and Fair IG -23** CG 59 HbA1c changes presented by % CG -9 Majority of patients in IG experienced 1-2 GI events of weight loss Percent of patients that decreased the amount that occurred early, of mild-moderate intensity, of oral sulfonylurea medication transient, and resolved spontaneously Other: note that unable to locate IG 43.2 Withdrew due to GI event , n weight change from CG 28.9 IG 7 randomization; give changes CG 2 during lead in Percent Withdrew due to adverse events, n Discontinued sulfonylurea medication IG 12 IG 11.7 CG 23 CG -- Other AEs: Flatus with discharge, oily spotting, fecal urgency, fatty/oily stool, oily evacuation, fecal N (percent) incontinence, increased defecation, vitamin D, E or Withdrew from trial prematurely because of β-carotene supplementation needed (due to 2 or elevated plasma glucose levels on 3 or more more consecutive low vitamin levels). No evidence occasions despite maximal sulfonlyurea for the development of gallstones or renal stones medication after orlistat treatment. Mean plasma levels of IG 5 (2.5) vitamins A, D, E and β -carotene remained within CG 15 (8.8) reference range through study. At 12 mo, mean vitamin E and β -carotene levels were lower in IG ** p=0.0019 than CG (p<0.001). No sig change in vitamin E to LDL ratio in either group. Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument Krempf, 2003193 NR NR Percent Subgroup analyses: Changes Withdrew prematurely due to AE in fasting glucose, LDL, HDL, Fair IG 6.9 triglycerides, SBP, and DPB CG 3.4 presented for those "at risk" per 1+ adverse event those same measures at BL, IG 86.1, however ns at BL for each CG 72.3, condition NR p<0.001 (Difference was because of the % of orlistat patients experiencing GI events, suggesting Other: NR fat intake was still excessive, although reduced from initial.) N Withdrew prematurely due to serious adverse events* IG 5 CG 4 * 7 of these events were deemed doubtfully related to study by investigators (IG: thrombotic thrombocytopenic purpura, anal abscess, pain hypocondrium, liver disorder; CG: abdominal pain, breast cancer, ulcerative colitis) Lindgarde, NR A higher proportion of IG patients with type 2 Percent Subgroup analyses: Weight 2000194 diabetes were able to stop or reduce their 12 mo change, fasting glucose, and dosage of anti-diabetic meds compared with Gastrointestinal events HbA1c in patients with type 2 Swedish CG (23.3% vs. 18.2%) IG 80 diabetes Multimorbidity CG 39 Study Overall incidence for other adverse events was Other: NR similar between IG and CG. 10 IG patients and 5 Fair CG patients withdrew due to an adverse event. 5 IG patients and 1 CG patient withdrew because of GI events. 19 IG patients and 5 CG patients experienced serious adverse events, none of which were considered by study investigators to have a probable causal relationship with the study medication. 1 death occurred in IG; patient had type 2 diabetes and severe arterio-sclerosis and died as a result of a brain stem infarction. Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument Miles, 2002197 NR Mean (SD) Percent Subgroup analyses: NR 12 mo 12 mo Fair Reduction in metformin dose, mg/day Experiencing at least one gastrointestinal event Other: NR IG -16 (24)* IG 83 CG 49 (24) CG 62 Reduction in relative sulfonylurea dose, %† Mild-Moderate hypoglycemic episodes IG -11.5 (3.6)* IG 10 CG -0.9 (2.6) CG 4 † Doses standardized to a % of maximum daily Withdrew due to adverse events, n dose IG 25 * p<0.05 CG 12 Twice as many patients in IG vs CG either reduced or discontinued 1 or more diabetes More IG than CG patients discontinued treatment medications (17.1 vs. 8.2%). More CG than IG because of an adverse event (10 vs. 5%, p<0.05) patients required additional or increased doses of diabetes medication (21.7 vs. 12.2%). These changes in diabetes medication usage were significantly different between groups (p=0.0004) Richelsen, NR n (percent) Percent Subgroup analyses: Dietary 2007198 BL 36 mo 36 mo intake for a subsample Newly developed Diabetes Mellitus Withdrawals due to adverse events (Svendsen) Fair IG -- 8 (5.2)* IG 5 CG -- 17 (10.9) CG 5 Other: Number (IG vs CG) of Fatty/oily stool patients who started with meds * p=0.041 IG 23 with statins (11 vs 11) metformin CG 2.5 (13 vs 18) blood pressure (84 vs Oily spotting 90) was same in 2 groups IG 17.5 CG 0 Abdominal pain IG 21.5 CG 16 Fecal urgency IG 8.5 CG 5 One or more gastrointestinal event IG 88* CG 63 Serious adverse event IG 18 CG 28 * p<0.01; statistical significance NR for first 5 AEs. Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument Rossner, QOL QOL 24 mo Subgroup analyses: Outcomes 2000199 Instrument used: IG1 and IG2 reported significantly greater Withdrew due to severe GI events, percent also reported for completers Technology satisfaction with their weight loss medication IG1 6.6 Fair Assessment versus CG after 1 and 2 years (p<0.001 for IG2, IG2 10.3 Other: NR Group quality-of- p<0.05 for IG1). IG2 patients also expressed CG 3.4 life questionnaire greater satisfaction both with losing weight and Withdrew due to adverse events, percent Range: NR their weight loss program (p=0.011 and IG1 9.6 # of questions: 55 p=0.002, respectively, after 2 years). Overall IG2 7.9 Directionality: NR satisfaction with treatment, as expressed by the CG 2.5 Description: treatment index, was significantly greater Withdrew due to adverse GI events, percent Measures obesity among IG1 and IG2 versus CG after 2 years IG1 5 distress, (p<0.001 for IG2, p<0.05 for IG1). IG1 and IG2 IG2 3.7 depression, patients reported less overweight distress than CG 0.8 satisfaction with CG and this became statistically significant after 2 serious adverse events possibly related to orlistat: treatment 2 years (p<0.05). There were no significant 1 case of cholelithiasis and diverticulitis. Adverse differences between treatment groups in event profiles were similar in all 3 groups (except GI NOTE: The study depression scores after 1 or 2 years events) throughout study, generally mild-moderate calls this QOL, in intensity and resolved spontaneously. Majority of but it is a QOL severe GI events occurred during year 1 (n=38). scale specific to Majority of vit-amin supplement occurred during obesity and might year 1. Differences in mean plasma values for not correspond vitamins D, E and β-carotene between IG1/IG2 and with other QOL CG were statistically significant (p<0.001). Orlistat instruments we had no clinical significant effects on pulse rate or have ECG results. Other AEs: Fatty/oily stool, fecal urgency, oily spotting, increased defecation, fecal incontinence, flatus with dis-charge, oily evacuation, vitamin supplement, breast ca Sjostrom, NR NR 12 mo Subgroup analyses: Bone 1998200 Adverse event frequency, % density measured for a very IG 94 small subsample (n=30) Fair CG 82 (Gotfredson, #8364) did not Premature withdrawals due to GI adverse events, show difference between IG and % CG in bone mineral IG 3.5 measurement during 1 year CG 0.6 Premature withdrawals due to other adverse Other: Authors found low events, % systemic absorption of orlistat IG 3.2 after 2 years of treatment with no CG 2.0 evidence of accumulation Frequency of adverse events slightly higher in IG vs CG in year 1 and similar for all 4 treatment groups in year 2. Patients taking orlistat experienced far fewer GI events in year 2 vs year 1. Serious adverse events reported by 24 CG patients and 25 IG in year 1, only 1 related to treatment. 2 adverse events in year 2 related to treatment. 1 case of GI neoplasm in CG. Events occurring in <5% of patients NR. Other AEs: Fecal incontinence, flatus with discharge, fecal urgency, abdominal pain, liquid/soft Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument stool, oily spotting, increased defecation, fatty/oily stool, oily evacuation, headache, 2 consecutive low vitamin A, D, E levels, vitamin supplementation, other reason
Swinburn, QOL Mean (SD) at BL, Mean change (SD) at 12 mo Percent Subgroup analyses: NR 2005201 Instrument used: SF-36 Physical functioning 12 mo SF-36 IG 75.5 (19.6) 3.23 (1.97) At least one adverse event Other: Change in medications Fair Range: 0-100 for CG 75.7 (19.5) 1.32 (18.0) IG 94.7 for diabetes mellitus, each domain SF-36 Physical role CG 93.5 hypertension, lipids in IG and CG # of questions: IG 78.8 (34.6) 1.41 (40.0) Serious adverse events shown in a figure only. NR CG 78.8 (33.4) 3.06 (32.2) IG 9.4 Directionality: SF-36 Bodily pain CG 7.1 Higher score = IG 72.1 (23.2) 0.70 (22.7) Gastrointestinal system adverse event better CG 75.1 (23.6) -2.33 (22.0) IG 82.4* SF-36 General health CG 60.4 IG 69.1 (19.6) 3.28 (14.8) Withdrew because of GI adverse events CG 70.1 (18.4) 0.13 (14.6) IG 2.9 SF-36 Vitality CG 1.2 IG 61.7 (19.8) 5.42 (19.3)* Withdrew because of adverse events CG 62.3 (19.4) -1.51 (19.4) IG 10.0 (calc) SF-36 Social functioning CG 4.7 (calc) IG 83.7 (23.4) 2.88 (24.0) CG 86.1 (20.7) -0.77 (25.7) * p=0.0005 SF-36 Emotional role IG 84.5 (31.9) 2.58 (36.8) In general, adverse events were mild to moderate CG 90.0 (23.9) -5.48 (31.8) in intensity. For all other events reported in more SF-36 Mental health than 10 participants in either group, there were no IG 77.9 (15.6) 3.15 (15.3) statistically significant differences between IG and CG 79.6 (15.7) -0.52 (17.9) CG. * p=0.006; There were significant changes toward fewer or lower-dose medications in IG for diabetes (p=0.026) and hypertension (p=0.0062), but not for lipids (p=0.42) Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument Torgerson, NR Cumulative incidence, percent Percent Subgroup analyses: Incidence 2004202 BL 4 yr 1 yr 4 yr of DM among pts with IGT at BL; Diabetes Mellitus 1+ gastrointestinal event HR of developing DM by BL Torgerson, IG 0 6.2** IG 91 36 glucose tolerance, sex, age, and 2001291 CG 0 9.0 CG 65 23 BMI; weight loss for completers Diabetes Mellitus among those with IGT at 1+ SAE, percent only, and for all randomized (BL XENDOS baseline IG -- 15 carried forward for dropouts); IG 0 18.8** CG -- 13 proportion weight loss ≥5% and Fair C 0 28.8 1+ serious gastrointestinal event ≥10%, and for completers only IG -- 2 Hazard ratio (95% CI) CG -- 2 Other: Other intermediate Risk of developing diabetes Withdrew due to AE or laboratory abnormalities outcomes only reported for 851 IG v. CG -- 0.63 (0.46, 0.87)** IG -- 8 and 567 pts in IG and CG IGT v. NGT -- 10.60 (7.30, 5.4)*** CG -- 4 respectively at 4 years Male v. Female -- 1.41 (1.02, 1.96)* Death >44 v. ≤44 years† -- 1.44 (1.02, 2.04)* IG -- 0 ≥37 vs. < 37 kg/m2† -- 1.36 (0.97, 1.91) CG -- 0
† Median Mean change from baseline *** p < 0.001 Vitamin A, µmol/L ** p < 0.01 IG -- -0.22* * p < 0.05 CG -- -0.19 25-hydroxyvitamin D, nmol/mL IG -- -17.2** CG -- -13.0 Vitamin E, µmol/L IG -- -2.8** CG -- 0.4 Vitamin K1, µg/L IG -- -0.08** CG -- -0.07 1,25-hydroxyvitamin D, pmol/mL IG -- -15.8 CG -- -14.0
Proportion that went from normal to having two subsequent, consecutive abnormally low values was similar for Vitamin A (5.5 vs 4.4%) and notably different only for Vitamin E (3.2 vs 0.5%). Proportion for all other vitamin levels were <1% and similar between treatment groups
* p<0.05 for IG vs CG ** p<0.001 for IG vs CG Metformin Trials Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument Fontbonne, NR During the course of the trial, no patient Reasons for absence at last visit, percent Subgroup analyses: Fasting 1996185 developed ischemic cardiovascular disease but 12 mo blood glucose by glucose 5 CG patients were diagnosed with diabetes by Side effect of allocated treatment tolerance at baseline BIGPRO local investigators IG 17.5 CG 4.3 Other: All participants weighed Fair Death every 3 months IG 1.6 CG 0 Diabetes IG 0 CG 2.9 Other health problems IG 7.9 CG 5.7
Other AEs: Diarrhea*, Nausea/vomiting, Abdominal pain, Constipation, Cramps, Headache/fatigue, Mood shifts, Cutaneous rash, Hunger, Bad taste in mouth
*Except for diarrhea and to a much lesser degree, nausea and vomiting, all other reported side effects occurred with similar frequence in both treatment groups Gambineri, NR N (percent) Two women who completed the study reported Subgroup analyses: NR 2006186 BL 7 mo 13 mo transient abdominal discomfort (abdominal Impaired fasting glucose swelling, mild diarrhea, and flatulence) during the Other: NR Fair IG 3 (15) 3 (15) 3 (15) first 2 weeks of treatment CG 2 (11) 1 (5) 2 (11) Impaired glucose tolerance IG 3 (15) 4 (20) 2 (10) CG 2 (11) 1 (5) 0 (0) Impaired fasting glucose + Impaired glucose tolerance IG 2 (10) 0 (0) 0 (0) CG 2 (11) 1 (5) 0 (0)
IG n analyzed: 20 CG n analyzed: 19 Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument Diabetes Depression BL 12 mo 48 mo (3.2 yrs for age groups) Subgroup analyses: Age, Prevention Instrument used: Depression: BDI ≥11 or antidepressant use (%) GI symptoms (diarrhea, flatulence, nausea, gender, race Program Beck Depression IG-men 8.1 8.6 vomiting), number of events/100 person-years Research Inventory or IG-wmn 19.7 14.7 Total Other: 10-year unblinded Group, 1999142 current use of CG-men 9.1 7.5 IG 77.8* followup results available antidepressants CG-wmn 18.1 17.1 CG 30.7 (#8173). Haffner, 2005212 (BDI ≥11 36 mo 25-44 years threshold used for Diabetes crude cumulative incidence, cases/100 IG 82.2 As has been previously observed Orchard, depression) p-y CG 32.4 with this drug, the IG participants 2005262 Range: NR Total 45-59 years experienced modest weight loss, # of questions: IG 7.8 IG 77.5 which was greatest in the oldest Diabetes NR CG 11.0 CG 30.8 age group. Waist circumference Prevention Directionality: 25-44 years 60-85 years was reduced, with the greatest Program Higher score = IG 6.7 IG 72.2 change in the 60-85 year age Research worse; used CG 11.6 CG 27.8 group. In contrast, there were no Group, 2006210 score ≥ 11 as 45-59 years Deaths, number/100 person-years significant changes in weight or threshold for mild IG 7.6 Total waist circumference at any age in Ratner, 2005207 depression CG 10.8 IG 0.20 the CG. ≥ 60 years CG 0.16 Knowler, Anxiety IG 9.6 25-44 years After removal of interaction terms, 2002206 Instrument use: CG 10.8 IG 0.11 race (p<0.0001) and gender Beck Anxiety Male CG 0 (p=0.0259) main effects were not West, 2008214 Inventory IG 8.1 45-59 years significant within metformin Range: 0-63 CG 12.5 IG 0.13 treatment. Rubin, 2005205 # of questions: Female CG 0 NR IG 7.6 60-85 years Metformin interventions produced Ackermann, Directionality: CG 10.3 IG 0.48 significantly larger percent weight 2009211 Higher score = White CG 0.86 loss than CG across the race- worse IG 7.8 gender groups (all p<0.05). The Diabetes CG 10.3 * p<0.05 for comparison with CG only exception to this pattern was Prevention QOL Black that Hispanic women within the IG Program Instrument used: IG 7.1 IG n analyzed: 1073 (22-44 yrs: 318; 45-59 yrs: did not experience significantly Medical CG 12.4 541; 60-85 yrs: 214) greater percent weight loss than Good Outcomes Study Hispanic CG n analyzed: 1092 (22-44 yrs: 324; 45-59 yrs: those in CG (p=0.0547). 36-item short IG 8.4 557; 60-85 yrs: 201) form (SF-36); can CG 11.7 The study had inadequate power be used to American Indian Gastrointestinal complaints were more common in to assess the significance of determine SF-6D, IG 9.7 IG (as expected), with rates slightly lower in the effects within the subgroups, nor MCS and PCS CG 12.9 middle-age and older groups, although this were such tests planned. scores Asian/Pacific Islander difference was not statistically significant. The rate Treatment effects did not differ Range: NR IG 7.5 of gastrointestinal symptoms was highest in IG. significantly according either to # of questions: 36 CG 12.1 Hospitalization and mortality rates were unrelated sex or race or ethnic group. Directionality: BL BMI 22 to <30 to treatment. No deaths were attributed to Lower score = IG 8.8 intervention. Effect of metformin was less with worse CG 9.0 a lower BMI or a lower fasting BL BMI 30 to <35 Other AEs: Musculoskeletal problems (mostly glucose concentration than with IG 7.6 myalgia, arthritis, arthralgia), Hospital admissions, higher values for those variables. CG 8.9 Rate of hospitalization, Hospital stay Diabetes incidence, % lower from CG (95% CI) IG 31 (17, 43)† Study Reference Health Outcome Health Outcomes Adverse Effects Comments Quality Rating Instrument (continued) QOL Nonfatal cardiovascular disease events, % Diabetes Instrument used: IG 1.7 Prevention Quality of Well- CG 1.5 Program Being Scale Nonfatal cardiovascular disease events, event Research (QWB-SA) rate (number of events per 1000 p-y) Group, 1999142 Range: NR IG 5.2 Haffner, 2005212 # of questions: CG 7.3 Orchard, NR Cardiovascular disease related deaths, n 2005262 Directionality: IG 1 Diabetes Higher score = CG 4 Prevention better Antihypertensive pharmacologic therapy Program prevalence, % Research IG 32 Group, 2006210 CG 31 Ratner, 2005207 **p<0.001 for IG vs CG Knowler, † Significant by group-sequential log-rank test 2002206 †† Diabetes incidence did not differ by age in CG West, 2008214 (11.0, 10.8, 10.3 cases per 100 p-y).Incidence in Rubin, 2005205 IG was lowest among youngest participants (6.7 Ackermann, vs 7.7 vs 9.3 cases per 100 p-y), but this trend 2009211 was not statistically significant (p=0.07). Diabetes 12 mo change from BL Prevention Anxiety, Beck Anxiety Inventory Program IG 3.75 (4.69) -0.15 (4.44) CG 3.78 (4.89) -0.25 (4.80) Good IG n analyzed: 1001 (BL), 992 (12 mos) CG n analyzed: 1012 (BL), 993 (12 mos) SF-6D IG 0.797 (0.105) -0.002 (0.108) CG 0.788 (0.111) -0.013 (0.106) SF-36, physical component score IG 50.1 (7.3) 0.22 (7.49) CG 50.4 (7.2) -0.04 (7.12) SF-36, mental component score IG 54.1 (7.7) -0.58 (8.30) CG 54.0 (7.4) -1.16 (8.33) IG n analyzed: 1067 (BL), 1011 (12 mos) CG n analyzed: 1079 (BL), 1018 (12 mos) Quality of Well-being, QWB-SA IG 0.693 (0.114) 0.017 (0.105) CG 0.700 (0.115) 0.013 (0.124) IG n analyzed: 707 (BL), 262 (12 mos) CG n analyzed: 702 (BL), 252 (12 mos) In a fully adjusted model including both IG and weight change, assignment to either IG was not significantly associated with change in SF-6D at 12 mo vs CG. After adjusting for IG, change in weight asscoiated with significant change at 12 mo for SF-6D (p<0.001), PCS-36 (p<0.001), MCS-36 (p=0.04) for ever 5 kg loss; similar associations at 24 mo Abbreviations: AE=adverse event; ANCOVA=analysis of covariance; AUC=area under the curve; BDI=Beck Depression Inventory; bid=two times a day; BL=baseline; BMI=body mass index; BMR=basal metabolic rate; BP=blood pressure; bpm=beats per minute; calc=calculated; carb=carbohydrate; CG=control group; CI=confidence interval; CV=cardiovascular; DBP=diastolic blood pressure; DM=diabetes mellitus; DPP=Diabetes Prevention Program; ECG=electrocardiography; GI=gastrointestinal; GP=general practitioner; HDL=high-density lipoprotein; HOMA=homeostatic model assessment; HR=heart rate; IG=intervention group; IGT=impaired glucose tolerance; IR=insulin resistance; ITT=intention to treat; IQR=interquartile range; LDL=low-density lipoprotein; LOCF=last observation carried forward; LSM=least squares mean; MA=meta-analysis; MI=myocardial infarction; n=number; NA=not applicable; NGT=normal glucose tolerance; NR=not reported; NYHA=New York Heart Association; OGTT=oral glucose tolerance test; PCOS=polycystic ovary syndrome; PCP=primary care practitioner; pt=patient; QOL=quality of life; RCT=randomized controlled trial; SAE=serious adverse event; SBP=systolic blood pressure; Sc=subcutaneous; SD=standard deviation; SE=standard error; SES=socioeconomic status; SF-36=36-Item Short-form Health Survey; TG=triglyceride; tid=three times a day; UK=United Kingdom; US=United States; VLCD=very low calorie diet; WC=waist circumference; WHO=World Health Organization.