Supplementary Tables 2005-03-03637
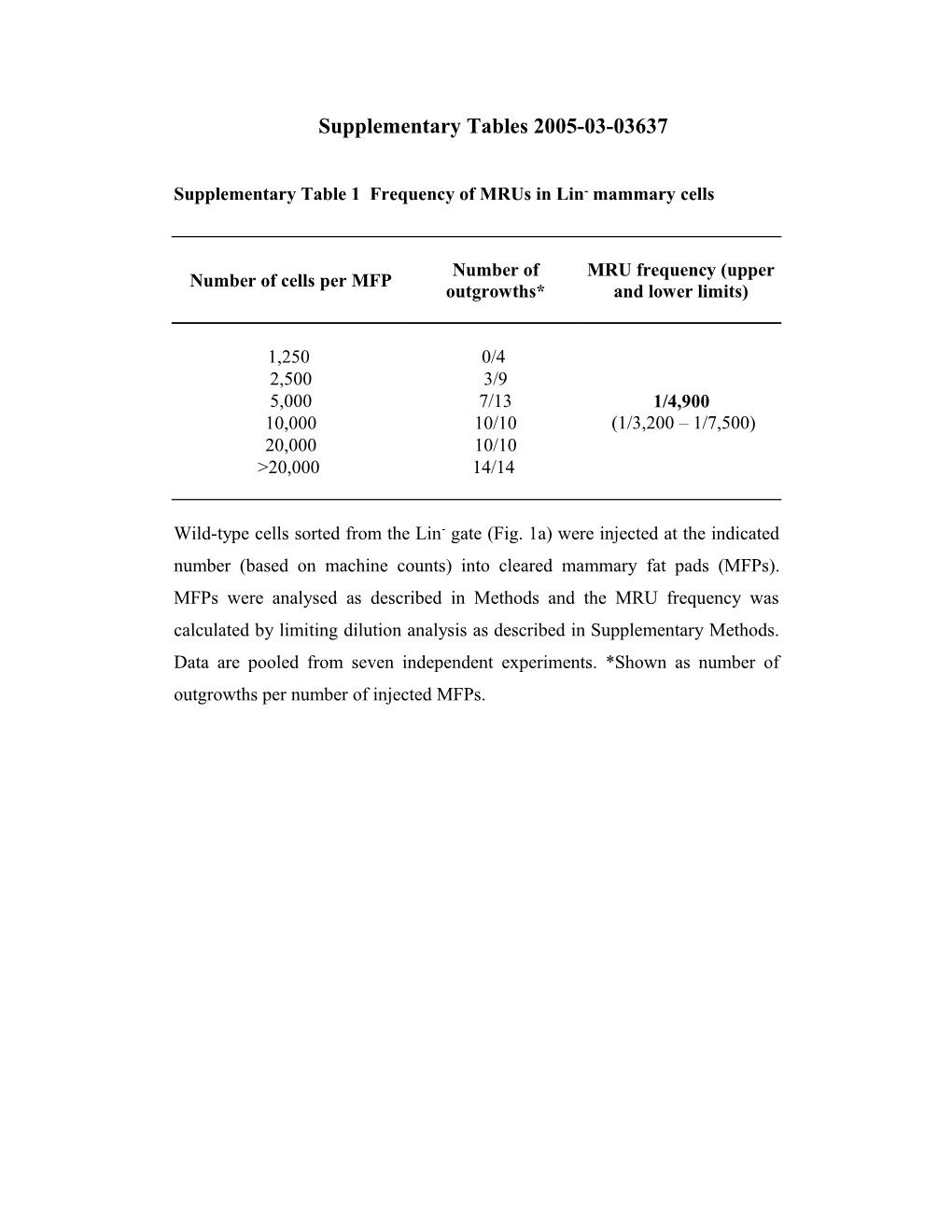
Supplementary Table 1 Frequency of MRUs in Lin- mammary cells
Number of MRU frequency (upper Number of cells per MFP outgrowths* and lower limits)
1,250 0/4 2,500 3/9 5,000 7/13 1/4,900 10,000 10/10 (1/3,200 – 1/7,500) 20,000 10/10 >20,000 14/14
Wild-type cells sorted from the Lin- gate (Fig. 1a) were injected at the indicated number (based on machine counts) into cleared mammary fat pads (MFPs). MFPs were analysed as described in Methods and the MRU frequency was calculated by limiting dilution analysis as described in Supplementary Methods. Data are pooled from seven independent experiments. *Shown as number of outgrowths per number of injected MFPs. Supplementary Table 2 Frequency of MRUs in subsets of Lin- mammary cells defined by CD29 and CD24 expression
Phenotype Number of cells Number of MRU frequency (upper per MFP outgrowths* and lower limits)
CD29lo CD24- 2,300 0/6 2,400 0/8 8,500 0/8 1/147,000 9,200 1/4 (1/37,000 - 1/590,000) 9,300 1/10 12,000 0/6
CD29lo CD24+ 190 0/6 220 0/8 400 0/7 <1/7,200 430 0/8 600 0/10 650 0/10
CD29hi CD24- 54 0/9 120 1/6 170 0/7 1/2,900 260 0/6 (1/1,100 - 1/7,800) 420 1/8 580 2/9
CD29hi CD24+ 18 0/5 20 0/9 60 2/7 1/590 100 3/11 (1/300 - 1/1,100) 200 3/10 200 1/11
Wild-type cells sorted from the four Lin- populations defined by CD24 and CD29 expression (Fig. 1c) were transplanted into cleared MFPs at numbers (based on machine counts) proportional to their frequency in the Lin- population, using the calculated MRU frequency of 1/5,000 for Lin- cells, according to the method described in Uchida and Weissman15. MFPs were analysed as described in Methods and MRU frequencies were calculated as described in Supplementary Methods. Data are pooled from six independent experiments. *Shown as number of outgrowths per number of injected MFPs.
2 Supplementary Table 3 High Sca-1 expression does not define a MRU-enriched subset of Lin- mammary cells
Number of cells Number of MRU frequency (upper Phenotype per MFP outgrowths* and lower limits)
Small Sca-1hi (R3) 2,100 0/6 2,300 0/5 1/30,000 3,300 2/13 (1/10,000 – 1/93,000) 3,600 1/8
Small Sca-1mid-lo (R4) 1,900 2/7 2,400 2/5 1/8,900 4,800 7/16 (1/5,100 – 1/16,000) 5,200 2/8
Large Sca-1 lo-hi (R5) 1,100 0/7 1,300 1/9 1/37,000 2,000 0/4 (1/5,200 – 1/260,000) 2,100 0/6
Mammary cells bearing the surface marker Sca-1/Ly6A/E have been reported to be enriched for progenitor activity in vivo7. We therefore assessed expression of this marker in the Lin-CD29hiCD24+ subpopulation by co-staining for Sca-1, CD29 and CD24, but found no significant Sca-1hi population within the Lin-CD29hiCD24+ gate (Fig. 1d). Cells sorted according to Sca-1 expression and size (Fig. 1d, gates R3-5) were transplanted into cleared MFPs and the MFPs analysed as described in Methods. The MRU frequency (see Supplementary Methods) was at least three-fold higher in the smaller-sized, Sca-1lo population than the Sca-1hi or large-sized populations. Data are pooled from three independent experiments and are displayed* as the number of outgrowths per number of injected MFPs. The high level of Sca-1 staining previously observed7 in mammary progenitor-enriched populations is likely to reflect the use of cultured mammary epithelial cells rather than the freshly isolated cells used here. In direct support of this, we found that Sca-1 expression was substantially elevated on mammary epithelial cells cultured for 3 days (data not shown).
3 Supplementary Table 4 Hoechst33342 exclusion does not define a MRU-enriched subset of Lin- mammary cells
Number of cells Number of MRU frequency (upper Phenotype per MFP outgrowths* and lower limits)
Hoechst-MP 2,400 3/6 2,800 1/3 3,700 3/4 1/2,900 5,700 4/4 (1/1,600 – 1/5,100) 11,000 4/4 15,000 4/4
Hoechst-SP 37 0/6 65 0/3 75 0/4 1/3,300 130 0/4 (1/470 – 1/23,000) 260 0/4 300 1/4
Several types of stem cells, but not all, have an increased ability to exclude dyes such as
Hoechst33342, due to the expression of membrane transporter proteins. In the mammary gland, the Hoechst33342 side population (SP) has been reported to be enriched for progenitor activity7,8. However, flow cytometric analysis of mammary cells co-stained
- with Hoechst33342, CD29 and CD24 revealed depletion of SP cells in the Lin CD29hiCD24+ gate (Fig. 1e). We further investigated this finding by transplantation (into cleared MFPs) of SP and main population (MP) cells (Fig. 1e) in numbers proportional to their frequency in the Lin- gate. MFPs were analysed as described in Methods and MRU frequencies calculated as described in Supplementary Methods. Data are pooled from three independent experiments and are displayed* as the number of outgrowths per number of injected MFPs. These experiments demonstrated that MP cells frequently gave rise to epithelial outgrowths while SP cells did not. We therefore conclude that the mammary SP fraction is not enriched in MaSCs, although some mammary progenitors may reside within it.
4 Supplementary Table 5 Frequency of MRUs in subsets of double-sorted, visualised Lin- mammary cells
Number of cells Number of MRU frequency (upper Phenotype per MFP outgrowths* and lower limits)
CD29lo CD24+ 100 - 109 0/6 110 - 119 0/9 120 - 129 0/6 <1/1,100 130 - 139 0/7 140 - 149 0/2
CD29hi CD24- 90 - 99 0/2 100 - 109 0/7 110 - 119 0/9 <1/1,100 120 - 129 0/7 130 - 139 0/4
CD29hi CD24+ 10 - 19 3/38 30 - 49 6/6 1/64 50 - 79 13/17 (1/46 – 1/90) 80 - 99 6/8 100 - 149 9/12
LacZ+ cells from the Lin-CD29loCD24+, Lin-CD29hiCD24- and Lin-CD29hiCD24+ populations were double-sorted, counted (with trypan blue) and injected at the indicated number into cleared MFPs. MFPs were analysed as described in Methods and MRU frequencies calculated as described in Supplementary Methods. *Shown as number of outgrowths per number of injected MFPs.
5 Supplementary Table 6 Mammary epithelial outgrowths derived from single Lin- CD29hiCD24+ cells
Supporting Number of cells outgrowths*
Cell suspension† - 3/33 + 5/35
Visualized single cells§ - 2/32 - 2/32 + 2/38
LacZ+ Lin-CD29hiCD24+ cells from Rosa-26 mice were double-sorted, counted, injected into cleared MFPs and the MFPs analysed as described in Methods. Cells were injected in 10 l volumes, either taken from a cell suspension containing 1 cell per 10 l† or in which a single cell had been prospectively visualized§ (see Methods). Results of independent experiments are shown. *Shown as number of LacZ+ outgrowths per number of injected MFPs.
6 Supplementary Table 7 In vivo self-renewal assays: secondary outgrowths derived from primary transplants of Lin-CD29hiCD24+ cells
Number of cells per Number of secondary primary transplant outgrowths*
25 17/18 24 7/16 23 12/12 22 4/18 14 11/18
1† 15/18§
Cell suspensions containing cells from LacZ+ outgrowths derived from primary transplantation of double-sorted, counted Lin-CD29hiCD24+ cells were detected by PCR as described in Methods, divided equally, and secondarily transplanted into cleared MFPs (~10,000 cells per transplant, including epithelial, endothelial, haemopoietic and stromal cells). MFPs were analysed as described in Methods. Results of independent experiments are shown. *Shown as number of secondary LacZ+ outgrowths per number of injected MFPs. †The primary outgrowth of single cell origin used for secondary transplantation was harvested from a recipient at parturition. All other primary outgrowths were harvested from virgin recipients. §All secondary outgrowths that derived from the single-cell origin primary outgrowth displayed full alveolar differentiation at parturition.
7