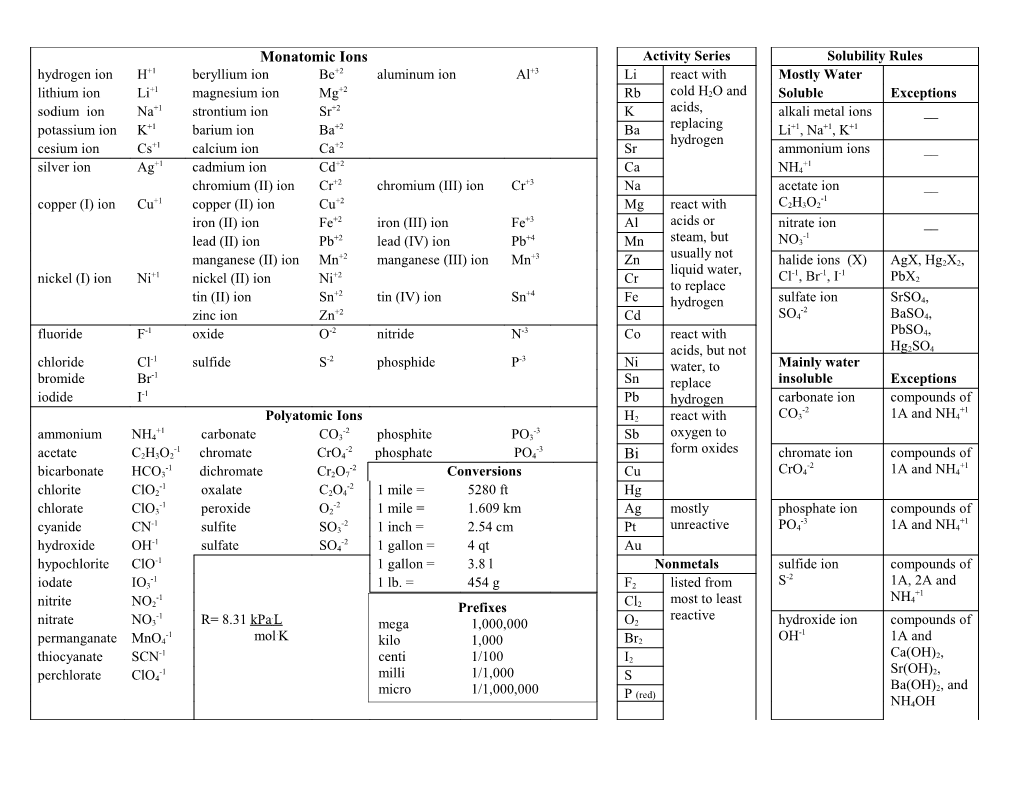
Monatomic Ions Activity Series Solubility Rules hydrogen ion H+1 beryllium ion Be+2 aluminum ion Al+3 Li react with Mostly Water +1 +2 lithium ion Li magnesium ion Mg Rb cold H2O and Soluble Exceptions sodium ion Na+1 strontium ion Sr+2 K acids, alkali metal ions __ potassium ion K+1 barium ion Ba+2 Ba replacing Li+1, Na+1, K+1 hydrogen cesium ion Cs+1 calcium ion Ca+2 Sr ammonium ions __ +1 +2 +1 silver ion Ag cadmium ion Cd Ca NH4 chromium (II) ion Cr+2 chromium (III) ion Cr+3 Na acetate ion __ +1 +2 -1 copper (I) ion Cu copper (II) ion Cu Mg react with C2H3O2 iron (II) ion Fe+2 iron (III) ion Fe+3 Al acids or nitrate ion __ +2 +4 -1 lead (II) ion Pb lead (IV) ion Pb Mn steam, but NO3 +2 +3 usually not manganese (II) ion Mn manganese (III) ion Mn Zn halide ions (X) AgX, Hg2X2, +1 +2 liquid water, -1 -1 -1 nickel (I) ion Ni nickel (II) ion Ni Cr Cl , Br , I PbX2 to replace +2 +4 tin (II) ion Sn tin (IV) ion Sn Fe hydrogen sulfate ion SrSO4, +2 -2 zinc ion Zn Cd SO4 BaSO4, fluoride F-1 oxide O-2 nitride N-3 Co react with PbSO4, acids, but not Hg2SO4 -1 -2 -3 chloride Cl sulfide S phosphide P Ni water, to Mainly water -1 bromide Br Sn replace insoluble Exceptions iodide I-1 Pb hydrogen carbonate ion compounds of -2 +1 Polyatomic Ions H2 react with CO3 1A and NH4 +1 -2 -3 ammonium NH4 carbonate CO3 phosphite PO3 Sb oxygen to -1 -2 -3 form oxides acetate C2H3O2 chromate CrO4 phosphate PO4 Bi chromate ion compounds of -1 -2 -2 +1 bicarbonate HCO3 dichromate Cr2O7 Conversions Cu CrO4 1A and NH4 -1 -2 chlorite ClO2 oxalate C2O4 1 mile = 5280 ft Hg -1 -2 chlorate ClO3 peroxide O2 1 mile = 1.609 km Ag mostly phosphate ion compounds of -1 -2 -3 +1 cyanide CN sulfite SO3 1 inch = 2.54 cm Pt unreactive PO4 1A and NH4 -1 -2 hydroxide OH sulfate SO4 1 gallon = 4 qt Au hypochlorite ClO-1 1 gallon = 3.8 l Nonmetals sulfide ion compounds of -1 -2 iodate IO3 1 lb. = 454 g F2 listed from S 1A, 2A and +1 -1 most to least NH4 nitrite NO2 Prefixes Cl2 -1 . reactive nitrate NO3 R= 8.31 kPa L mega 1,000,000 O2 hydroxide ion compounds of -1 . -1 permanganate MnO4 mol K kilo 1,000 Br2 OH 1A and -1 Ca(OH)2, thiocyanate SCN centi 1/100 I2 -1 Sr(OH)2, perchlorate ClO4 milli 1/1,000 S Ba(OH)2, and micro 1/1,000,000 P (red) NH4OH
Activity Series
Total Page:16
File Type:pdf, Size:1020Kb
Recommended publications