Sam Clark Period 6 10/1/09 Osmosis and Diffusion Lab Report
DATA COLLECTION AND PROCESSING
Raw Data Hard copy turned in
Processed Data
Part A Individual Data Contents in Initial Final Mass % Change in Class average % Beaker Mass Mass Difference Mass change in mass Distilled 13.0g 14.0g 1.0g 7.69% 15.0% Water
Class Data Contents Group 1 Group 2 Group 3 Group 4 Group 5 6th Junior Expected in Period Class Results Beaker Average Average Distilled 13.8% 18.8% 7.69% 20.0% 13.8% 15.0% 21.4% Water 0.2 M 5.66% 7.55% 11.1% 9.89% 10.5% 8.6% 6.9% 0.4 M 6.38% 12.96% 3.70% 3.7% 5.8% -4.5% 0.6 M -21.8% -21.6% -30.9% -13.9% -22.4% -22.5% -12.8% 0.8 M -25.9% -21.2% -25.5% -25.6% -25.5% -24.6% -23.0% 1.0 M -17.9% -22.4% -32.7% -30.2% -30.7% -31.2% -26.8% -23.5%
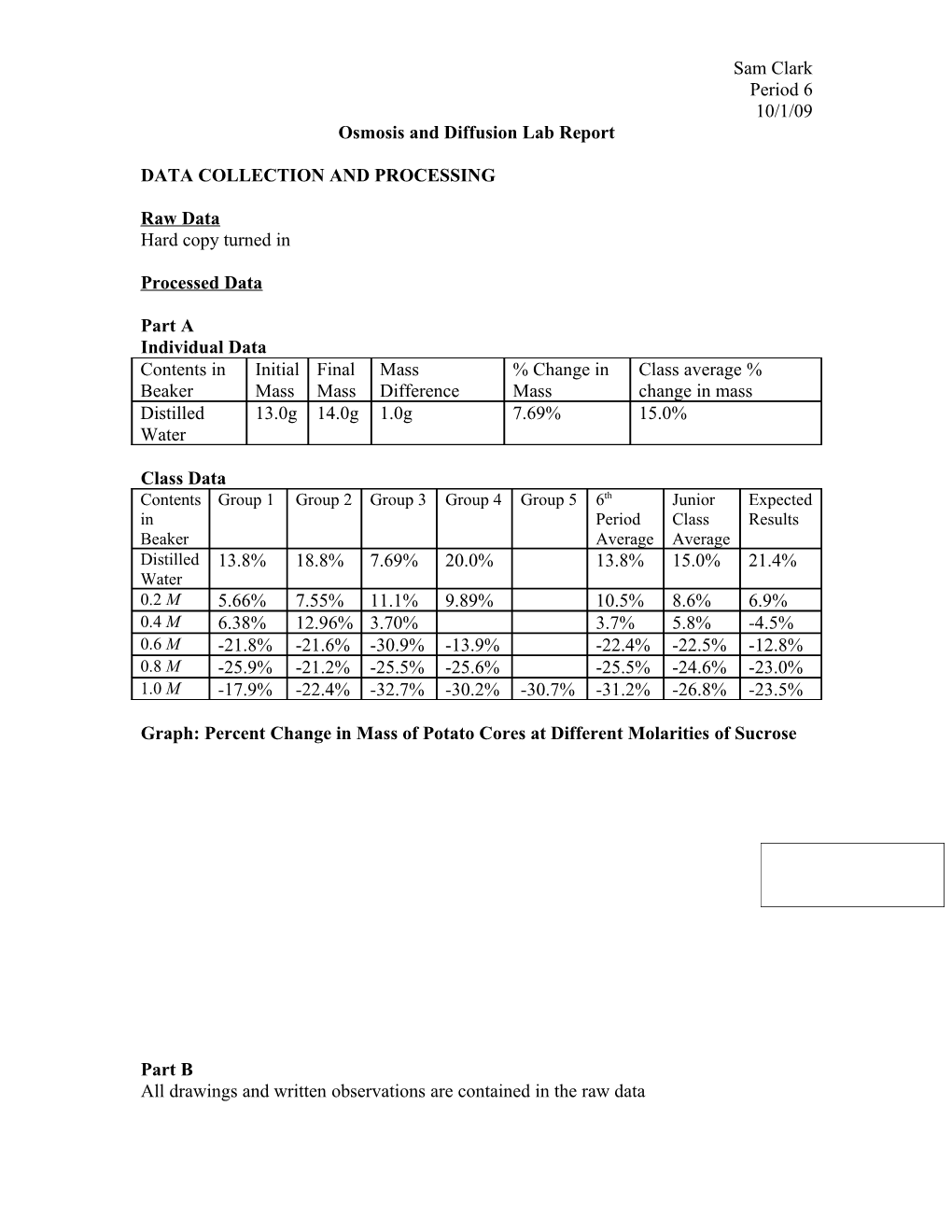
Graph: Percent Change in Mass of Potato Cores at Different Molarities of Sucrose 30 20 10
0 Expected Values -10 Distilled 0.2 M 0.4 M 0.6 M 0.8 M 1.0 M Junior Class Average Water Sucrose Sucrose Sucrose Sucrose Sucrose -20 Period 6 Ave. -30 -40 -50
Part B All drawings and written observations are contained in the raw data Sam Clark Period 6 10/1/09
Part C Individual Data Contents in Initial Mass Final Mass Mass % Change in Dialysis Bag Difference Mass Distilled Water 24.5g 24.2g -0.3g -1.22%
Class Data Contents Group 1 Group 2 Group 3 Group 4 Group 5 6th Junior Expected in Dialysis Period Class Results Bag Average Average Distilled -4.16% 0.0% 0.0% -1.22% -0.61% -1.35% 1.2% Water 0.2 M 0.0% 4.0% 4.4% 1.53% 2.97% 2.48% 3.1% 0.4 M 7.69% 8.00% 8.63% 11.1% 9.87% 8.86% 7.7% 0.6 M 9.09% 6.8% 11.72% 11.72% 9.20% 11.0% 0.8 M 10.78% 11.1% 16.0% 16.0% 16.0% 13.47% 14.8% 1.0 M 14.8% 19.05% 8.21% 12.0% 20.0% 13.40% 14.81% 18.2%
Graph: Percent Change in Mass of Dialysis Bags at Different Molarities of Sucrose 40 35 30 25 20 Expected Values Junior Class Average 15 Period 6 Ave. 10 5 0 -5 Distilled 0.2 M 0.4 M 0.6 M 0.8 M 1.0 M Water Sucrose Sucrose Sucrose Sucrose Sucrose
Errors and Uncertainties
Part A In Part A, the 6th period and junior class averages for percent change in potato core mass were fairly consistent with expected results for 0.2, 0.8, and 1.0 M sucrose solutions, but varied somewhat drastically for the other solutions. The most drastic discrepancy was 0.4 M solution, which had a positive change in mass in both sets of averages, but was expected to in fact lose mass. It would be reasonable to assume that errors could occur from the overly dried out or overly wet nature of the potatoes, but because both averages were very different for distilled water, 0.4, and 0.6 M sucrose solutions, it would make more sense that problems existed due to the mixing of these solutions. The molarity of Sam Clark Period 6 10/1/09 sucrose in these solutions was likely different than intended, yielding different results as water diffused into and out of the potato quite differently given the molarity of the solution. No other specific errors can be identified in terms of procedure, as not every group could be observed by one group in one period, but it is also quite possible that errors in procedure or the confusion of solutions occurred for some groups.
Part B No errors observed, as the data is not quantitative.
Part C Recorded percent changes were much more similar to expected percent changes in Part C than in Part A, as only distilled water and 1.0 M solutions had considerably different results from those expected. In distilled water, the bag lost mass in both averages when it was expected to gain mass, leading one to believe that perhaps the tap water in the dialysis bag had a slightly lower molarity than the water in which the bag was immersed. This would compel the more pure water in the bag to diffuse into the beaker. The discrepancies in 1.0 M solution were likely due to individual group error in procedure, as Groups 1, 2, and 5 were close to the expected result, but Groups 3 and 4 were not, therefore bringing the overall averages down. Inconsistencies could also be due to error in drying off the dialysis bags after removal from the beakers, as failure to adequately do so would yield an inaccurate mass.
CONCLUSION AND EVALUATION
Part A In Part A, the potato cores gained mass when immersed in distilled water, 0.2 M solution, and (according to the recorded data, not expected results) 0.4 M solution, and lost mass in 0.6 M solution, 0.8 M solution, 1.0 M solution, and (according to expected results) 0.4 M solution. This was a clear demonstration of osmosis, the specific instance of diffusion in which water moves from an area of high concentration to an area of low concentration. Because the potato cores in distilled water gained mass, it is clear that the potatoes had a lower concentration of water than simple distilled water, and therefore gained substantial mass as water diffused into the potato. Likewise in water with a 0.2 molarity of sucrose, the concentration of water was still higher in the solution than in the potato core. At 0.4 or 0.6 M solution (depending on which results are used, this will be discussed when discussing limitations and weaknesses), however, the potato begins to lose mass which proves that, knowing the principle of osmosis, the potato has a higher concentration of pure water than a solution with a 0.4 or 0.6 molarity of sucrose. The same applied for 0.8 and 1.0 M solutions, as potatoes in both of these solutions loss significant mass, indicating that there was much more pure water in the potato than in the solutions, as the pure water diffused out of the potato when moving from low to high concentrations. As mentioned when outlining errors and uncertainties, there was a clear discrepancy between class average data recordings and expected results regarding percent change in distilled water, 0.6 M, and especially 0.4 M solutions. Because the discrepancies occurred rather specifically for these three solutions, it is clear that a Sam Clark Period 6 10/1/09 fundamental weakness in the experiment lay in the mixing of solutions, which ended up being these three. 0.4 M solution likely had much, much less sucrose than it should have had, seeing as it yielded a gain in mass for the potato rather than a loss in mass, distilled water likely had more sucrose than it should have, and the 0.6 M solution likely had more sucrose than it should have, given that the potato lost much more mass than it should have. This weakness would have been alleviated first by simply ensuring more diligently that the solutions were of the correct molarity. Multiple trials in which each group mixed its own solution would also have helped improve the investigation, as not all the data for a certain molarity would be affected by a single inaccurate mixture.
Part B In Part B, onion cells were observed when immersed in distilled water, then in a solution of 15% NaCl, and finally when flooded with more water. This part of the experiment also clearly demonstrated diffusion in action. When immersed in distilled water, the onion cells, dyed in pink, were normal with pigment evenly distributed throughout all of the cells. When the NaCL solution was introduced, however, the cell membranes shriveled (the cell walls maintained unmoving) as the liquid within the cell membranes diffused outward. The cells became flooded with a solution with a lower concentration of pure water, and therefore the water within the cell moved outward, from high to low concentration. This shows why the pigment became splotchy and more scattered, as the membranes shrank due to the diffusion of water out of the membranes. When the cells were again flooded with distilled or tap water, the pigment appeared to swell again, as the water diffused back into the cells, swelling the membranes. This occurred because, with water having diffused from the cells, the water that now flooded the cells was of a higher concentration than the water within the cells, and therefore diffused inward. There were no incredibly serious weaknesses or limitations to this section of the lab, as all activities of diffusion in the cell could be easily observed, which was in fact the primary, if not the sole, objective of this part of the lab. All limitations would rest only in human error at not being able to properly operate a microscope or cutting off too thick of an onion sample to adequately observe cell activity. No such errors occurred, and if they did, were easily remedied mid experiment. But overall, this qualitative data collection had no fundamental weaknesses or limitations, given its objective.
Part C Part C led to essentially the same conclusions as Part A, in regards to osmosis of pure water from high concentration to low concentration. The expected results and, for the most part, the recorded results, followed a consistent trend. Solutions of higher sucrose molarity in the dialysis bag gained more mass than solutions of lower molarity gained, but all solutions with a sugar molarity gained mass. This is because, as was illustrated in both parts A and B, water moves from high to low concentration. The distilled water in the beaker had more pure water than any of the molar solutions in the dialysis bags, and therefore the water diffused across the bag’s semipermeable membrane into the bag, giving it more mass. When the dialysis bag contained distilled water just like the beaker, it was expected that it would gain mass, as there is simply, volume-wise, more water in the beaker. Sam Clark Period 6 10/1/09 Unlike the mentioned expected results for distilled water, the recorded data for distilled water indicated a loss in mass for the dialysis bag, clearly indicating that somehow the molarity of the water in the beaker was higher than the molarity of the water in the dialysis bag. There does not seem to be any reasonable explanation for this, leading one to believe that there were simply group inconsistencies in weighing— perhaps, as described in errors and uncertainties, the bags were not dried off before weighing, or the bags leaked, or the scales were not properly tared in a given group. These are not fundamental limitations or weaknesses within the experiment, but rather human error. Limitations and weaknesses can however be identified, as the 1.0 M solution averages were very consistently quite different from the expected results, while other solution averages were fairly close. Because of how consistent this discrepancy is, it likely indicates again a weakness in the 1.0 M solution itself, which was likely not properly mixed. This problem could be fixed in the same manner that the weaknesses from Part A could be fixed: with more diligence in mixing, or with multiple trials with each group mixing its own solutions, thus preventing one poorly mixed solution from altering an entire set of data, as it did here and in Part A.