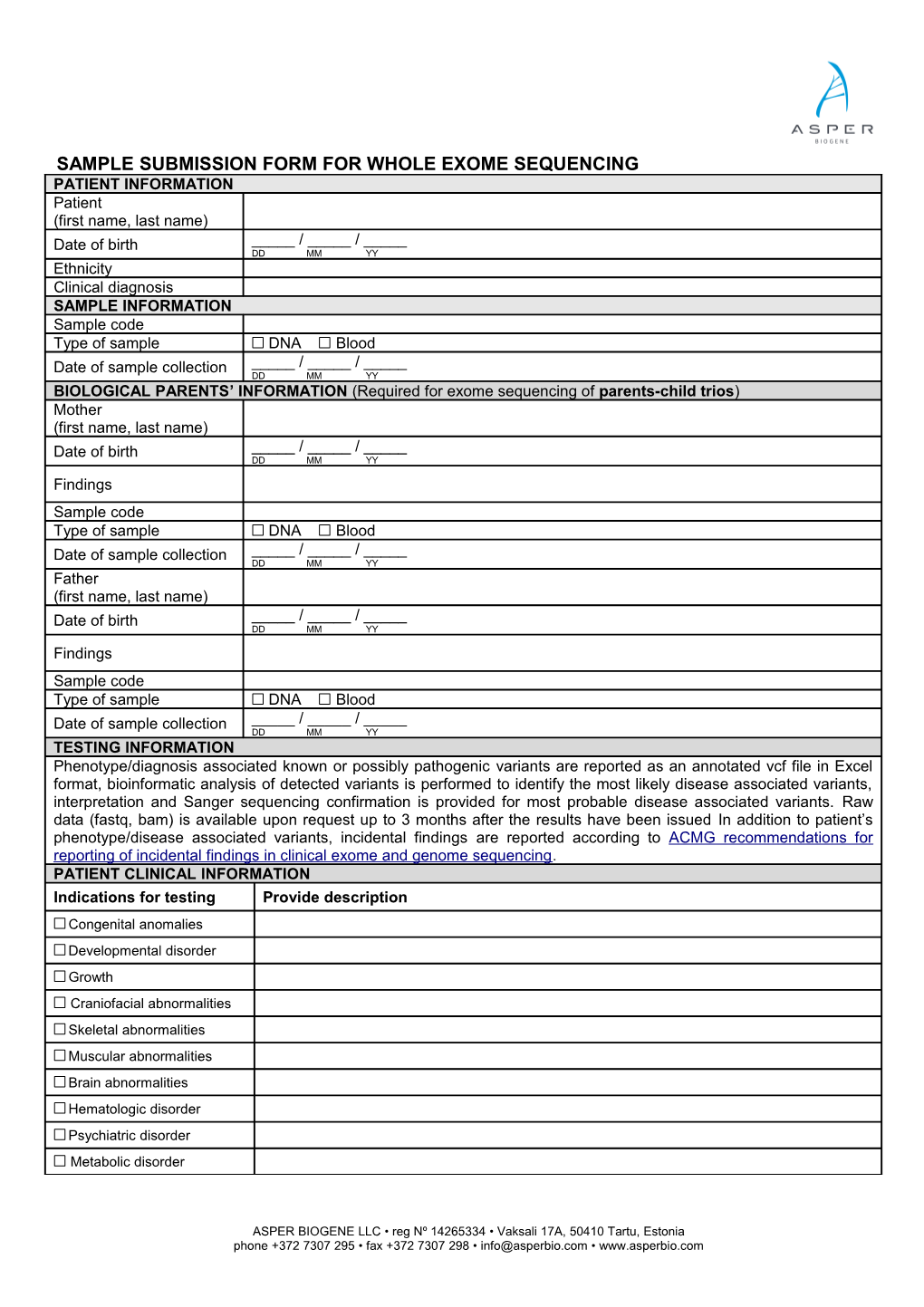
SAMPLE SUBMISSION FORM FOR WHOLE EXOME SEQUENCING PATIENT INFORMATION Patient (first name, last name) Date of birth _____ / _____ / _____ DD MM YY Ethnicity Clinical diagnosis SAMPLE INFORMATION Sample code Type of sample DNA Blood Date of sample collection _____ / _____ / _____ DD MM YY BIOLOGICAL PARENTS’ INFORMATION (Required for exome sequencing of parents-child trios) Mother (first name, last name) Date of birth _____ / _____ / _____ DD MM YY Findings Sample code Type of sample DNA Blood Date of sample collection _____ / _____ / _____ DD MM YY Father (first name, last name) Date of birth _____ / _____ / _____ DD MM YY Findings Sample code Type of sample DNA Blood Date of sample collection _____ / _____ / _____ DD MM YY TESTING INFORMATION Phenotype/diagnosis associated known or possibly pathogenic variants are reported as an annotated vcf file in Excel format, bioinformatic analysis of detected variants is performed to identify the most likely disease associated variants, interpretation and Sanger sequencing confirmation is provided for most probable disease associated variants. Raw data (fastq, bam) is available upon request up to 3 months after the results have been issued In addition to patient’s phenotype/disease associated variants, incidental findings are reported according to ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. PATIENT CLINICAL INFORMATION Indications for testing Provide description Congenital anomalies Developmental disorder Growth Craniofacial abnormalities Skeletal abnormalities Muscular abnormalities Brain abnormalities Hematologic disorder Psychiatric disorder Metabolic disorder
ASPER BIOGENE LLC • reg Nº 14265334 • Vaksali 17A, 50410 Tartu, Estonia phone +372 7307 295 • fax +372 7307 298 • [email protected] • www.asperbio.com Indications for testing Provide description
ASPER BIOGENE LLC • reg Nº 14265334 • Vaksali 17A, 50410 Tartu, Estonia phone +372 7307 295 • fax +372 7307 298 • [email protected] • www.asperbio.com Genitourinary abnormalities Dermatologic disorder Optical disorder Cardiac disorder Immunologic disorder Gastrointestinal disorder Otologic disorder Endocrine disorder Cancer formation
Other findings REQUESTING PHYSICIAN INFORMATION Requesting Physician (first name, last name) Hospital/Lab/Institution Samples receipt/order Person confirmation E-mail Address Results to be sent to E-mail Phone Results delivery by e-mail by regular mail Duplicate results Person to be sent to Address (if applicable) E-mail PAYMENT OPTIONS By submitting DNA samples to Asper Biogene, the client agrees that invoices will be paid within 10 calendar days as of the invoice date and, in case of a delay in the payment, open invoice amounts accrue interest amounting to 0.1% per calendar day. Institutional billing Contact information person Institution Address In EU countries, add the E-mail VAT account number of Phone the paying institution, VAT account otherwise 20% of VAT number tax will be added to the PO number invoice. Invoice by e-mail by regular mail delivery Patient’s data is needed for yes no invoicing
Authorization to use remaining sample material and test results Asper Biogene may use de-identified (without personal identifying information) remaining sample material and test
ASPER BIOGENE LLC • reg Nº 14265334 • Vaksali 17A, 50410 Tartu, Estonia phone +372 7307 295 • fax +372 7307 298 • [email protected] • www.asperbio.com results for quality improvements and/or scientific purposes.
I give my consent to use my de-identified sample material and test results as described above I do not give my consent to use my de-identified sample material and test results as described above
Name of patient……………………………………………………………………………………………………………………… Patient’s signature…………………………………………………………………………………………………………………… Date……………………………………………………………………………………………………………………………………
Important: By sending samples and placing an order customer accepts the Terms and Conditions of Asper Biogene (see website for details).
ASPER BIOGENE LLC • reg Nº 14265334 • Vaksali 17A, 50410 Tartu, Estonia phone +372 7307 295 • fax +372 7307 298 • [email protected] • www.asperbio.com