Page 1 of 4
Adverse Drug Events (ADE) Prevention Strategies and Gap Analysis
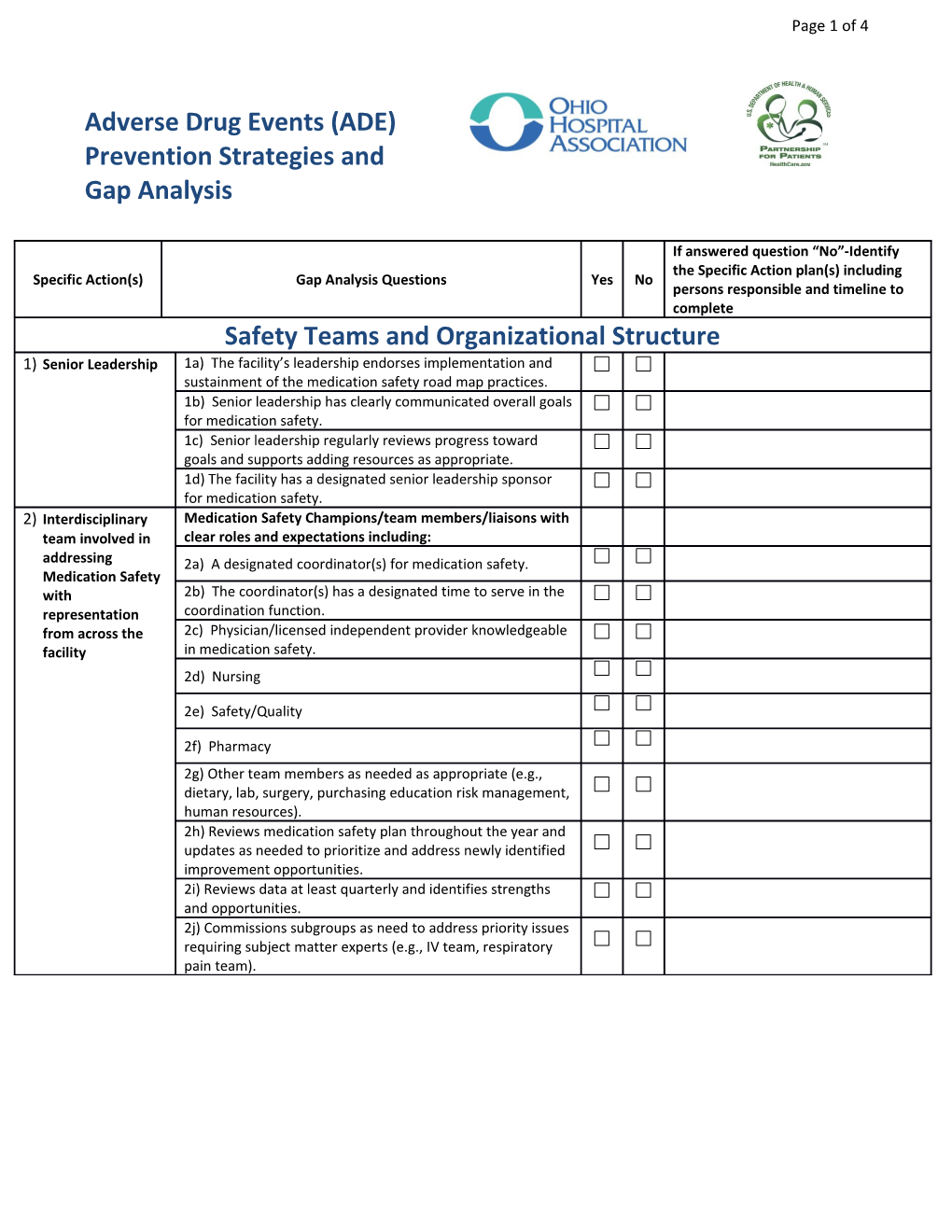
If answered question “No”-Identify the Specific Action plan(s) including Specific Action(s) Gap Analysis Questions Yes No persons responsible and timeline to complete Safety Teams and Organizational Structure 1) Senior Leadership 1a) The facility’s leadership endorses implementation and sustainment of the medication safety road map practices. 1b) Senior leadership has clearly communicated overall goals for medication safety. 1c) Senior leadership regularly reviews progress toward goals and supports adding resources as appropriate. 1d) The facility has a designated senior leadership sponsor for medication safety. 2) Interdisciplinary Medication Safety Champions/team members/liaisons with team involved in clear roles and expectations including: addressing 2a) A designated coordinator(s) for medication safety. Medication Safety with 2b) The coordinator(s) has a designated time to serve in the representation coordination function. from across the 2c) Physician/licensed independent provider knowledgeable facility in medication safety. 2d) Nursing
2e) Safety/Quality
2f) Pharmacy 2g) Other team members as needed as appropriate (e.g., dietary, lab, surgery, purchasing education risk management, human resources). 2h) Reviews medication safety plan throughout the year and updates as needed to prioritize and address newly identified improvement opportunities. 2i) Reviews data at least quarterly and identifies strengths and opportunities. 2j) Commissions subgroups as need to address priority issues requiring subject matter experts (e.g., IV team, respiratory pain team). Page 2 of 4 If answered question “No”-Identify the Specific Action plan(s) including Specific Action(s) Gap Analysis Questions Yes No persons responsible and timeline to complete Access to Information 3) Processes in place A process is in place to collect medication safety to access bundle/process date for the following as applicable: information for 3a) Hypoglycemic agent gap analysis data collection, analysis and 3b) Anticoagulant gap analysis sharing 3c) Opioid gap analysis Processes are in place to track the following ADE measures, at a minimum: 3d) Number of patients with INR > 5 (or outside of established therapeutic range.) 3e) Number of patients with blood glucose < 40 (or outside of established therapeutic range.) 3f) Number of naloxone administrations (or established opioid ADE measure.) 3g) A process in place to affirm the reliability of both the process and the outcome obtained through audits. 3h) Standard criteria exists for conducting audits (e.g., chart audits) when needed. 3i) The facility’s documentation system (electronic or paper) is designed to capture sufficient detail about ADEs that occur to allow for adequate event analysis. The facility has the following processes in place to analyze data including: 3j) Routinely review and analyze data for process improvement opportunities/defects. 3k) Analyze data related to possible medication-related readmissions to identify gaps and opportunities for improvement. 3l) Track progress against established targets (e.g., run charts, control charts, dashboards, scorecards.) 3m) Prioritize and act upon identified issues. The facility has processes in place to share ADE data on a regular basis including: 3n) Within units
3o) Across units
3p) With leadership
3q) With medical staff
3r) With the Board 3s) Provide stat lab results 24/7 to ensure safe and timely monitoring of high risk medications. Page 3 of 4
If answered question “No”-Identify the Specific Action plan(s) Specific Action(s) Gap Analysis Questions Yes No including persons responsible and timeline to complete Access to Information The facility’s EMR: 3t) Interfaces with the lab system to automatically alert practitioners to abnormal values, indicating a potential need to modify high-alert medication therapy. 3u) Screens medication therapy against the patient’s clinical profile for contraindications, interactions and dose appropriateness before drugs are administered. 3v) Alerts health care practitioners to duplicate class orders for medications. 3w) Performs dose range checks. 3x) Screens for and documents existing diseases or conditions that could affect the dosing of medication therapy prior to initiating antithrombotic, hypoglycemic or opioid therapy. Facility Expectations 4) Clear expectations 4a) The facility follows standardized clinical practice guidelines regarding policies, when developing order sets, policies and procedures. procedures and staff 4b) There is a standard process in place to promptly retrieve expectations outdated protocols, pathways, guidelines, order sets and/or supporting checklists throughout the facility and to replace with updated medication safety versions. 4c) There is a standard process in place to communicate changes to staff. 4d) Incorporate medication safety into new employee orientation. 4e) Educate staff annually on medication safety.
4f) Expectations supporting medication safety is included in new physician orientation . 4g) Evaluate staff competencies related to high-risk medications.
4h) The facility has structured communication tools for communication related to high-risk medications (i.g., SBAR) during hand-offs. 4i) During admission process, identification of: i. patient’s home med list
ii. potential drug interactions
iii. concomitant therapies
iv. verification of patient’s meds and dosages
v. flag potential for medication-related readmissions (i.g., polypharmacy, high-alert medications, multiple disease states, etc.) Specific Action(s) Gap Analysis Questions Yes No If answered question “No”-Identify the Specific Action plan(s) including persons Page 4 of 4 responsible and timeline to complete Facility Expectations 4j) A process is in place to promptly inform families when an ADE occurs and includes at a minimum:
i. direction on who should discuss the unanticipated outcome with the patient/family and how that discussion should occur. ii. individuals designated to provide disclosure to patients receive training on effective disclosure strategies.
iii. a process for disclosing to, and updating, patient/family as the event is reviewed and analyzed.
iv. a designated person is available to provide support and just-in-time training to staff members who are about to disclose an unanticipated outcome to a patient/family. Patient and Family Engagement 5. Involve Patient 5a) A process is in place to assess and address any barriers to and Family patient/family ability to understand their role in ADE prevention Regarding ADEs (e.g., cultural, hearing impairment and health literacy.) 5b) Patients/families are educated on their respective role in preventing ADEs and prevention measures they can expect to see from staff and providers caring for them in the hospital (e.g., bedside barcoding, explaining purpose of meds, identifying potential side effects, asking name and birthdate before med administration.) 5c) A process in place to assess patient/families’ level of understanding of the education provided (e.g., teach back.) 5d) The facility has a process in place to encourage patients and families to speak up if they have concerns about direct care/support staff/ provider practices or other issues that may increase the risk of medication error. 5e) A process is in place to report back to patients/families that have a shared concern.