Name: ______Hour: ______
Chemistry A Matter Labs (Labs that matter?) Name: ______Hour: ______
BrainPOP Measuring Matter Watch the video “Measuring Matter” on brainpop.com Username- greenwichps Password- gps2009
1. Matter is anything in the universe that takes up ______.
2. Mass, volume and density are ______we can take of matter.
3. We measure things in order to understand it and ______it to other stuff.
4. Standard metric units: for length ______for volume ______and for
mass______.
5. Mass is a measurement of how much ______an object contains and we measure it with a
______or scale. Name: ______Hour: ______6. Volume is a measure of how much ______something takes up. Solid volume is
measured in ______and is calculated length X ______X ______
7. Liquid volume is measured in ______. 1 mL = 1 cm3
8. Before dropping the rock into it the water had a volume of 50 mL, after dropping the rock in the
water level rose to 70 mL. What is the volume of the rock? ______
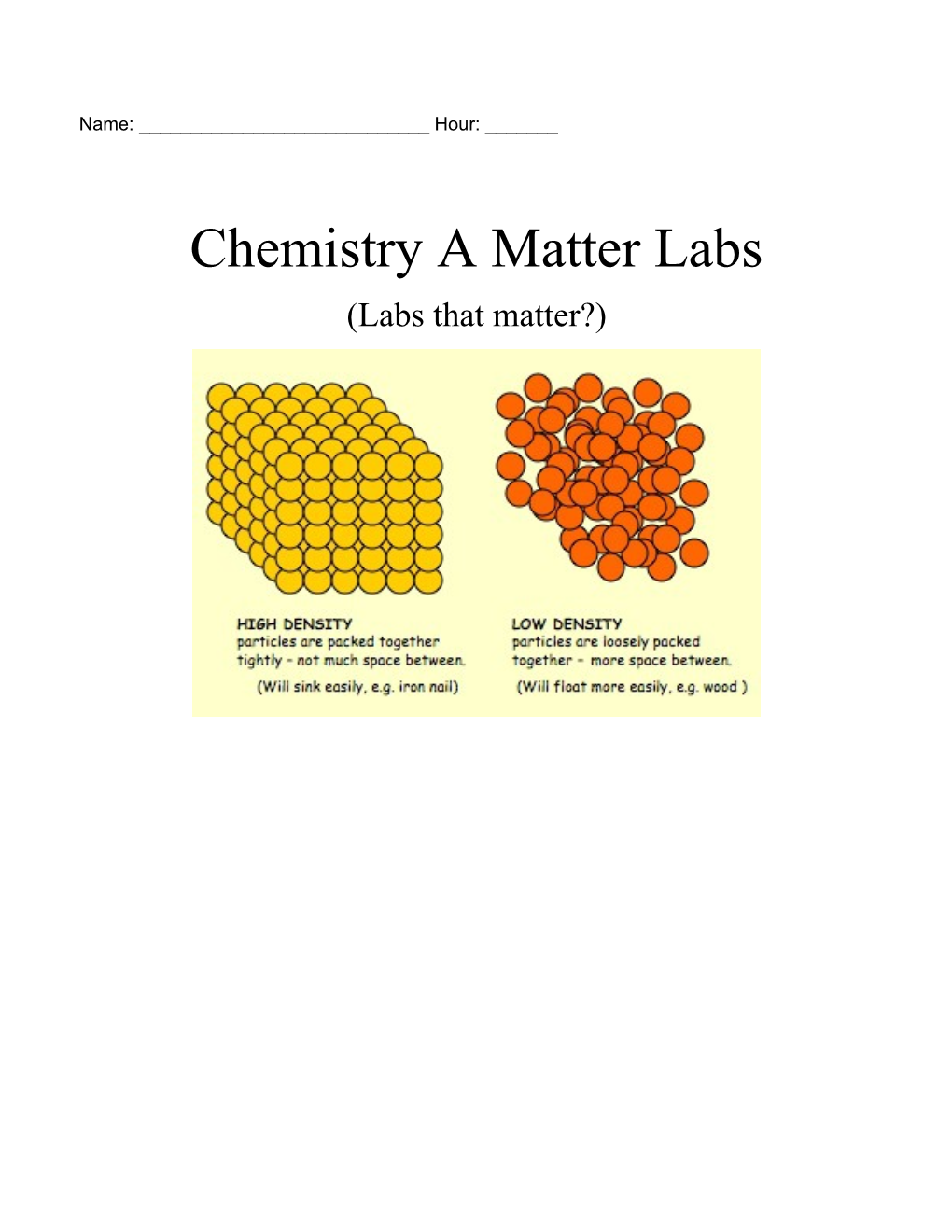
9. Density is how ______something is for its ______.
10. Density = ______
11. What are the units of density?
Measurement of Mass and Volume
Purpose: Make accurate measurements of mass and volume. Materials: metric ruler 25 mL graduate triple-beam balance water wooden box
Part One : Measuring Volume of a Uniform Solid Use a ruler to measure the area of the top of the box at your lab station. Your measurements must be in centimeters & include 2 places after the decimal. Length ______. ______units: ______
Width ______. ______units: ______
Height ______. ______units: ______
Calculate the volume of the box below. Show work and include 2 places after the decimal. Hint: Volume = length x width x height Units: cm3
Part Two : Measuring Volume of a Liquid Fill a graduated cylinder about half way full with water from the tap. Name: ______Hour: ______Record the volume of water. Your measurement must be in milliliters and include 1 place after the decimal. *LEAVE THE WATER IN THE CYLINDER.*
Volume of the water ______. ___ units: ______
Part Three : Measuring Mass Place the graduated cylinder including the water on the balance. Start with the 100g, carefully slide the masses until the pointer is level. Masses must be in a notch or your measurement is meaningless. Record the mass in grams and include 3 places after the decimal. *LEAVE THE WATER IN THE CYLINDER.*
Mass of the water______. ______units: ______
Part Four : Measuring Volume of a Non-Uniform Solid Place five aluminum pellets into the graduated cylinder that still contains the water you added. Record the new volume of the water. Your measurement must be in milliliters and include 1 place after the decimal. Volume of water and aluminum pellets ______. ___ units: ______
Now calculate the volume of the aluminum pellets. Initial volume of water (Part 2) minus new volume of the water (Part 4).
Volume of the aluminum pellets ______. ___ units: ______
Part Five : Clean Up 1. Make sure the ruler is at your lab station, remove the graduate and water from the balance. 2. Slide the masses on the balance to the left so that the balance is zeroed. ______3. Make sure you have your lab sheet and pencil. Teacher Initials
Follow Up Questions:
1. Define each of the following terms using the information in the front of this packet.
matter: ______
volume: ______
mass: ______
density: ______Name: ______Hour: ______
Look at your lab data to help you with the following questions
2. What units are used for each of the following measurements?
length: ______volume: ______or ______mass: ______
3. How many decimals do we round to when using the following tools?
ruler: ______graduated cylinder: ______triple beam balance: ______
4. Density = mass ÷ volume. Considering your answers to Question #2, what are the units for density? (Hint: It will be a fraction combining two units)
5. Did we determine the mass of the water in this lab? If so, what is it?
6. Did we determine the volume of the water in this lab? If so, what is it?
7. What additional measurement(s) we would need to make in order to calculate the density?
Density of Water Lab
Purpose: Use a buret to measure volume and determine the density of water.
Materials: 50 mL beaker waste container buret & clamp distilled water
Pre-Lab Questions 1. What are the units of mass? ______What are the units of volume of a liquid? ______2. When measuring mass with a balance, how many decimals do you round to? ______3. When measuring volume of a liquid with a graduated cylinder, how many decimals do you round to? _____ 4. What is the formula for density?
Procedure: 1. Find and record the mass or your empty, dry beaker. 2. Be sure your buret contains 50.0mL of water and that the bottom of the meniscus is on the 0.0 mL mark. 3. Allow water to flow from the buret into the beaker until you have added exactly 10.0 mL to the beaker. 4. Measure and record the mass of the beaker and water. 5. Do not empty the water from the beaker. Return the beaker to the buret and allow water to run into the Name: ______Hour: ______beaker until the buret reads 25.0 mL which means you now have a total of 25.0 mL of water in the beaker. 6. Measure and record the mass of the beaker and water. 7. Do not empty the water from the beaker. Return the beaker to the buret and allow water to run until the buret reads 40.0 mL. 8. Measure and record the mass of the beaker and water. 9. Finally return to the buret and allow water to run until you have 50.0 mL in the beaker. Stop the buret at 50.0 mL. Do not let water keep running down past the number line. 10. Measure and record the mass of the beaker and 50.0 mL of water. 11. Drain the rest of the water from the buret into the waste beaker and discard it in the sink. Make sure the balance is set back to zero and the pan is clean and dry. Wipe the area of the lab table where you worked to make sure it is clean and dry. TEACHER INITIALS ______Data and Calculations 10.0 mL of Water mass of beaker and water ______.______units _____ mass of empty beaker - _____.______units _____ Mass of water ______.______units _____
Density of water ______.___ units _____
25.0 mL of Water mass of beaker and water ______.______units _____ mass of empty beaker - ______.______units _____ Mass of water ______.______units _____
Density of water ______.___ units _____
40 mL of Water mass of beaker and water ______.______units _____ mass of empty beaker - ______.______units _____ Mass of water ______.______units _____
Density of water ______.___ units _____
50 mL of Water mass of beaker and water ______.______units _____ mass of empty beaker - _____.______units _____ Mass of water ______.______units _____
Density of water ______.___ units ____ Name: ______Hour: ______Graphing Density: Use the grid below to make a graph of your data. Since controlled for the volume that will be your independent variable (x) and the mass will be your dependent variable (y). Title: ______
The slope of a graph tells you how steep the graph is. Slope = (y2 – y1) ÷ (x2 –x1) Pick two points on your graph that are far away from each other, for example you might choose the 10.0 mL and
50.0 mL points. The first point will be (x1 , y1) and the second point will be (x2 , y2)
1. Calculate the slope of your graph, showing all your work and including units.
2. Notice that in the calculation above, we are dividing mass by volume. In this specific graph, what does the slope represent besides just how steep the graph is?
Conclusions: The density of water is 1.00 g/mL.
3. How close were your calculations of density (on the front) to the true value? If they were not close, explain any possible reasons why.
4. How close was the slope of your graph (in question #1 above) to the true value? If it was not close, explain any possible reasons why. Density of Aluminum Lab Purpose: Compare the density of two different forms of solid aluminum metal (aluminum strip and pellet)
Materials: Triple Beam Balance, Aluminum Strip, Metric Ruler, Aluminum Pellets, Graduated Cylinder, Water
Hypothesis: Which will have a greater density: the aluminum strip or the aluminum pellets? ______
Pre Lab Questions 1. What are units of mass? ______2. What are two possible units of volume? ______or ______a. When measuring volume of a uniform solid, which units should you use? ______b. When measuring volume of a non-uniform solid, which units should you use? ______3. When measuring volume with a ruler, how many decimals do you round to? ______4. When measuring volume of a liquid with a graduated cylinder, how many decimals do you round to? ____ 5. To find the volume of the aluminum strip, will you use a ruler or a graduated cylinder? ______6. To find the volume of the aluminum pellets, will you use a ruler or a graduated cylinder? ______
Trial #I: Determining the density of an aluminum strip (a uniform solid) 1) Make sure your balance is clean, dry and at zero. Name: ______Hour: ______2) Obtain an aluminum strip. 3) Using the balance determine the mass and record below. Report your answer to three decimals. 4) The thickness of the aluminum is 0.04cm. Record in the space below. 5) Using a ruler, determine the length and width of the aluminum strip in centimeters. Be sure to report your answer to two decimal places.
Mass of aluminum strip ____.______units ____ Thickness of aluminum strip 0.04 cm Width of the aluminum strip ____.__ __ units ____ Length of aluminum strip ____.__ __ units ____
Calculations:
1) Volume = Length X Width X Thickness (cm3). Using the data above, calculate volume and report your answer to two decimal places—even if your last number is a zero! SHOW YOUR WORK!
2) Density = mass ÷ volume. Using the mass you measured and recorded above and the volume you calculated in the above problem, determine the density of your aluminum strip in grams per cubic centimeter (g/cm3). Report your answer to two decimal places. SHOW YOUR WORK! Name: ______Hour: ______Trial #2: Determining the density of aluminum pellets (a non-uniform solid)
1) Add 12-16 aluminum pellets directly on the balance pan. 2) Record the mass of the aluminum to three places after the decimal. 3) Add distilled water to a graduated cylinder until you have a volume right around 25 mL. 4) Record this initial volume of water to one decimal place. 5) Carefully, without splashing, add the pellets to the cylinder of water. 6) Read and record the final volume of water and pellets. 7) Pour out the water and put the aluminum pellets on a paper towel on your table. DO NOT DUMP PELLETS INTO THE SINK.
Mass of aluminum ____.______units ___ Volume of pellets + water ____.__units ____ pellets Volume of water only -____.__units ____ Volume of pellets only ____.__units ____
Calculations :
1) Calculate the density of the aluminum pellets in grams per milliliter: Report answer to one decimal place.
Comparing Density of Aluminum Strip vs. Aluminum Pellets To do this we will calculate percent error for each trail. The smaller the percent error the closer your results are to the accepted value. The formula we use to calculate this is:
% error = observed value – expected value x 100 expected value
The observed value is what you calculated in Trials #1 and #2. The expected value for aluminum (strip and pellets) is 2.70 g/cm3.
2) Calculate your percent error for Trial # I (aluminum strip). Show all work and report your answer to two places past the decimal. Trial #1 % error = Trial #1 Answer – 2.70 X 100 = ______%
2.70 Name: ______Hour: ______Name: ______Hour: ______3) Calculate your percent error for Trial #2 (aluminum pellets). Show all work and report your answer to two places past the decimal. Trial #2 % error = Trial #2 Answer – 2.70 X 100 = ______%
2.70
4) What does it mean if you have a negative percent error? ______
5) What does it mean if your percent error is zero? ______
6) How close were you to the expected value of 2.70 g/cm3?
a. In Trial #1 I was (circle one: over/under) by ______
b. In Trial #2 I was (circle one: over/under) by ______
5) Was your hypothesis found to be correct? Explain. Name: ______Hour: ______
Comparing Density Graphs
Purpose: Compare the densities of a variety of materials by observing graphs.
Materials: Triple Beam Balance, Lead, Zinc, Aluminum, Brass, Block of Wood, Graduated Cylinder, Ruler
Pre- Lab Questions:
1. Define each of the following terms:
matter: ______
volume: ______
mass: ______
density: ______
2. What units are used for each of the following measurements?
mass: ______volume: ______or ______density: ______or ______
3. How many decimals do we round to when using the following tools?
ruler: ______graduated cylinder: ______triple beam balance: ______
4. Think back to the Density of Aluminum Lab: Why did you measure the density of the aluminum strip differently than the density of the aluminum pellets?
5. Think back to the Density of Aluminum Lab: What does the slope of a volume vs. mass graph tell us?
Find the Density: 1. Find the mass of each object using the triple beam balance. 2. Record your findings in the table below. Don’t forget the units. 3. To find the volume of the objects: a. Put about 30 ml of water in the graduated cylinder AND record the exact amount in the space labeled “Initial Volume”. b. Drop the object into the cylinder and record the total mL in the space labeled “Total Volume”. This is the volume of the water and the volume of the object. c. Find the difference between Initial and Total. Record in the column marked “Volume.”
Object Mass Volume of Volume of Volume of Density of Metal of Object Water Water + Metal (show your work) Metal
Lead
Zinc Name: ______Hour: ______
Aluminum
Brass
Why didn’t we use the formula, volume=length X width X height, to find the volume of the above objects?
Density of a Block of Wood: Find the mass, volume and density of wood. Show your work and include units and correct number of decimals.
Mass= ______units: ______
Volume=______units: ______
Show work for volume density calculation below:
Follow Up Questions: 1. Considering your work above, why does wood float on water? Hint: Think about the Density of Water lab.
2. Consider the graph to the right: a. What is the independent variable?
b. What is the dependent variable?
c. What does the slope of this graph represent?
d. Which of the three elements in the graph has the greatest density?
e. Which of the three elements has the smallest density?
3. In your lab you calculated the density of brass in addition to lead, zinc and aluminum. ESTIMATE where you would expect the line for brass to be and draw it in on the graph above. In other words- DO NOT plot points, just think about where the line would be relative to the lines that are already on the graph. Name: ______Hour: ______
4. Directly proportional means that as one value increases, the other value also increases. Inversely proportional means that as one value increases, the other value decreases. Do mass and volume appear to have a direct or an inverse relationship?