Teaching-Learning Program: Chemistry 2A/BCHE
1 CHEMISTRY YEAR 11 2012
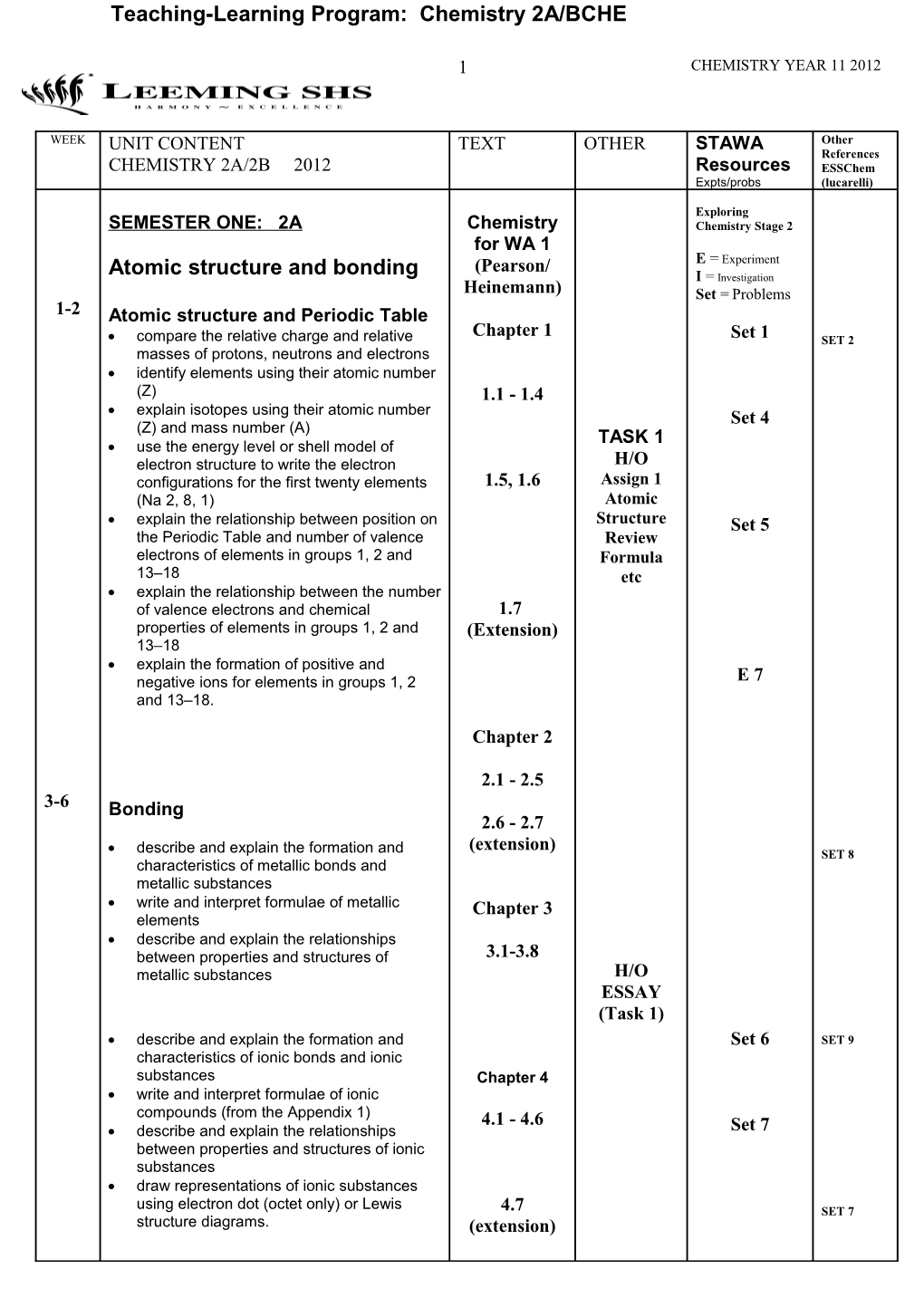
WEEK UNIT CONTENT TEXT OTHER STAWA Other References CHEMISTRY 2A/2B 2012 Resources ESSChem Expts/probs (lucarelli)
Exploring SEMESTER ONE: 2A Chemistry Chemistry Stage 2 for WA 1 (Pearson/ E = Experiment Atomic structure and bonding I = Investigation Heinemann) Set = Problems 1-2 Atomic structure and Periodic Table Chapter 1 compare the relative charge and relative Set 1 SET 2 masses of protons, neutrons and electrons identify elements using their atomic number (Z) 1.1 - 1.4 explain isotopes using their atomic number Set 4 (Z) and mass number (A) TASK 1 use the energy level or shell model of electron structure to write the electron H/O configurations for the first twenty elements 1.5, 1.6 Assign 1 (Na 2, 8, 1) Atomic explain the relationship between position on Structure Set 5 the Periodic Table and number of valence Review electrons of elements in groups 1, 2 and Formula 13–18 etc explain the relationship between the number of valence electrons and chemical 1.7 properties of elements in groups 1, 2 and (Extension) 13–18 explain the formation of positive and negative ions for elements in groups 1, 2 E 7 and 13–18.
Chapter 2
2.1 - 2.5 3-6 Bonding 2.6 - 2.7 (extension) describe and explain the formation and SET 8 characteristics of metallic bonds and metallic substances write and interpret formulae of metallic Chapter 3 elements describe and explain the relationships between properties and structures of 3.1-3.8 metallic substances H/O ESSAY (Task 1) describe and explain the formation and Set 6 SET 9 characteristics of ionic bonds and ionic substances Chapter 4 write and interpret formulae of ionic compounds (from the Appendix 1) 4.1 - 4.6 describe and explain the relationships Set 7 between properties and structures of ionic substances draw representations of ionic substances using electron dot (octet only) or Lewis 4.7 SET 7 structure diagrams. (extension) 2 CHEMISTRY YEAR 11 2012
describe and explain the formation and characteristics of covalent bonds covalent Chapter 5 molecular substances write and interpret formulae of covalent 5.1-5.3 molecular elements and compounds describe and explain the relationships between properties and structures of covalent molecular substances draw representations of molecular TASK 2 substances using electron dot (octet only) or SET 10 Lewis structure diagrams. ( completion 5.4 of set tasks atomic structure and Sets write the chemical formulae for molecular bondins) 8-11 compounds based on the number of atoms of each element present as inferred from the systematic names write the molecular formulae of commonly encountered molecules that have non- systematic names
describe and explain the formation and characteristics of covalent bonds covalent network substances SET 11 write and interpret formulae of covalent SET 12 network elements and compounds Chapter 6 TASK 3 describe and explain the relationships between properties and structures of 6.1-6.8 covalent network substances
Applied chemistry describe the relationships between properties and uses of ionic, metallic, covalent network and E 8 covalent molecular substances found in E 9 and around the home I 4
Chapter 7 7-8 Stoichiometry SET 13 perform simple calculations: 7.1-7.5 Set 12 1. molar mass (Qualitative 2. mole to mole only) I 5 3. mass to mole I 6 4. mass to mass SET 14 5. percentage composition E 11 Macroscopic properties of matter 9.8 9-10 Kinetic Theory use the Kinetic Theory of Matter to explain SET 1 1. relationship between heat and Set 3 temperature 3 CHEMISTRY YEAR 11 2012 2. change of phase Set 18 3. vapour pressure and factors that affect vapour pressure 4. effect on gases of changes in Set 19 pressure, temperature and volume 5. the characteristics of gases TASK 4 predict the effect on gases of changes in Chapter 8 Investigatio pressure, temperature and volume n: I3 or I6 (qualitative only) explain the boiling point of a liquid. 8.1-8.8 Stoichiometry perform simple calculations: 1. mass to volume (gases at S.T.P) 2. volume to volume 3. volume (at S.T.P) to moles
use molar volume of gases at STP in calculations involving the evolution of gases predict the effect on gases of changes in pressure, temperature and volume (qualitative only) explain the boiling point of a liquid. Set 2 TEST 2 Part1 STOICHIOMETRY and KINETIC THEORY 11-12 Set 15 SET 19 Solutions describe the characteristics of homogeneous and heterogeneous mixtures E 3 distinguish between pure substances, E 4 homogeneous mixtures and E 5 heterogeneous mixtures. Chapter 9 identify, explain and give examples of saturated, unsaturated and supersaturated I 2 solutions I 3 SET 20 apply solubility rules to predict if a 9.1 - 9.6 precipitate will form when two dilute ionic solutions are mixed (see data sheet) use the colour of ions (see data sheet) to identify reactants and the products in 9.7 chemical processes (extension) explain the effect of concentration on vapour pressure, melting point and boiling point of a solution describe the characteristics and give examples of strong, weak and non- electrolytes explain the differences between concentrated and dilute solutions of strong 12-13 and weak electrolytes. SET 21 Stoichiometry E 12 perform simple calculations involving concentration I 7 calculations (moles per Litre, grams per 4 CHEMISTRY YEAR 11 2012 Litre) TASK 5 Applied chemistry STAWA Calc describe and give examples of solutions Sets and their uses in and around the home Test explain concentration units used in Sample household mixtures (g 100g-1, mL L-1, g mL- 1, percentage composition)
EXAM WEEK 15/16
Chemical reactions
Reactions, equations and stoichiometry SET 6
write equations for simple chemical TASK 6 Test reactions (molecular or ionic) using state Chapter 10 symbols where appropriate. SET 17 explain conservation of mass, atoms and 10.1 - 10.5 charge during a chemical reaction. SET 22 perform stoichiometric problems that interrelate mass, molar mass, number of moles of solute, and concentration and volume of solution.
Applied chemistry investigate a biological, environmental or industrial process applicable to a chosen Chapter 11 context e.g. chemicals in the garden, kitchen chemistry or chemistry of cleaning. Include: 11.1 - 11.3 1. a description of the chosen process 2. an explanation of relationships between the chosen process and chemical TASK 7 models and theories 3. where appropriate: Report: safe handling and disposal of any materials or specific chemicals involved in the E 14 process E 15 discussion of sustainability of the process TASK 8 I 8 Investigatio n: Rates of TEST 2 Part 2 Reaction: Clock SOLUTIONS & STOICHIOMETRY Reaction. (Or selection I8 E 16 17- – I10) E 17 E 18 SET 15 19 Energy effects
use the Law of Conservation of Energy to explain endothermic and exothermic 5 CHEMISTRY YEAR 11 2012 reactions apply the concepts of system and surroundings to energy transfer Unit 2A explain enthalpy (H) in terms of stored Revision chemical energy TASK 9 explain endothermic and exothermic Practical reactions in terms of bond breaking and Test 1 bond making interpret and explain enthalpy diagrams and equations that include the heat lost or I 8- I 11 gained TASK 10 (selection) Applied chemistry describe and explain common examples of endothermic and exothermic reactions or processes in and around the home e.g. combustion, hot packs, change of phase
Reaction rates describe the rate of a reaction in terms of SET 16 rate of change of a measurable quantity with time TASK 11 identify and apply the factors affecting rates of reaction: 1. concentration 2. catalysts 3. temperature 4. state of sub-division apply the collision theory to explain the TASK 12 factors affecting rates of reaction Practical draw and interpret energy profile diagrams to show the transition state, activation Reports energy, uncatalysed and catalysed pathways and the heat of reaction (Due on explain the relationship between collision completion theory, kinetic energy distribution graphs of each and the rate of a reaction. experiment)
Applied chemistry describe and explain examples where rates of reaction have been altered in and around the home investigate real world problems in a laboratory setting with appropriate teacher direction, considering: 1. sources of uncertainty in experimental measurements 2. selection of the appropriate units of measurement of quantities such as volume and time
TEST 3 ENERGY AND RATES OF REACTION 6 CHEMISTRY YEAR 11 2012
SEMESTER TWO: 2B
19-20 Chemical reactions Chapter 12
Reactions, equations and stoichiometry Set 16 12.1-12.5 write equations that show only the species involved in the reaction perform calculations involving limiting reagent. empirical formula Set 17 molecular formula
21-23 Acids and bases in aqueous solutions Chapter 13
describe, explain and apply an understanding of the Arrhenius and Brønsted-Lowry models of acids and 13.1 - 13.8 TASK 4 E 19, 20 bases Assign Acids describe and explain the difference between strong /Bases acids including HCl, H2SO4, HNO3 and weak acids including CH3COOH and H3PO4 Set 21 identify acids by: 1. indicator colour Set 22 2. pH scale value 3. reaction with: o metal carbonates and hydrogen carbonates o metals such as magnesium and iron o metal oxides E21 – 24 o metal hydroxides (selection) describe and explain the difference between strong bases including group 1 and group 2 hydroxides and weak bases including NH3 and Na2CO3 identify bases by 1. indicator colour 2. pH scale value 3. reaction with: TASK 1 o acids Investigation: o ammonium salts (Selection: write equations for the successive ionisation of I11 – I13) Set 23 polyprotic acids describe, write equations and predict observations for the following reaction types: 1. acid-base 2. acid-carbonate 3. acid-metal qualitatively apply the pH scale I 12 – I 13 describe properties and reactions of non-metal and (selection) metal oxides e.g. reaction of SO2 with water.
Applied chemistry describe and explain the formation and impact of acids in the environment e.g. rain, acid rain, soil acidification in TASK 3 agriculture or acidification of ground water by mineral sulfides. TEST 4 ACIDS, BASES, STOICHIOMETRY 7 CHEMISTRY YEAR 11 2012
24-27 Set 24 Oxidation and reduction Chapter 14 explain oxidation and reduction as an electron 14.1 - 14.4 transfer process TASK 2 calculate oxidation numbers Assign Redox identify and name oxidants and reductants in equations identify oxidation-reduction reactions using oxidation numbers describe, write equations for and interpret Set 25 observations for: metal displacement reactions o E 25 o halogen displacement reactions write balanced simple redox equations (metal/metal E 26 ion, metal/hydrogen ion and halogen/halide ion) describe and explain how an electric current is Set 26 conducted in an electrolytic cell Chapter 15 describe and explain the following during the operation of an electrolytic cell: 15.1 - 15.5 E 27 o anode processes o cathode processes o role of the electrolyte o direction of ion migration o direction of electron flow in external circuit o electrode product prediction for molten metal o halides only. 60
Applied chemistry 61 predict and name the electrode products for the electrolysis of molten metal halides only. TASK 6 describe electro-winning and electro-refining Test 62 investigate real world problems in a laboratory setting with appropriate teacher direction, considering: o sources of uncertainty in experimental measurements o selection of the appropriate units of measurement of quantities such as volume and time
TEST 5 REDOX AND ELECTROLYSIS
28-30 Chapter 16 TASK 5 Assign Org
Organic chemistry 16.1-16.2 E 29 8 CHEMISTRY YEAR 11 2012 describe the bonding capacity of carbon explain the diversity of carbon based compounds E 30 Alkanes: Set27 name and draw straight and simple branched to C8 observations and equations for: 16.3 o substitution reactions o combustion reactions draw and name structural isomers
Alkenes: name and draw straight and simple branched to C8 (only one double bond per structure) 16.4 write observations and equations for: o addition reactions with halogens and hydrogen o combustion reactions draw and name structural and geometric isomers
Cycloalkanes and Cycloalkenes:
draw and name simple structures to C8 Set 28 write observations and equations for: 16.5 o substitution and combustion reactions for cycloalkanes o addition and combustion reactions for Set 29 cycloalkenes
Benzene: explain the unique structure and reactivity of benzene 16.6 write equations for: o catalysed substitution reactions with halogens o combustion reactions
TASK 7 Investigation: I14 (Chemical composition of Rocks
31-32 TASK 8 Practical Test 2 REACTIONS Applied chemistry
describe and explain the sources and uses of hydrocarbons e.g. fuels
perform calculations involving empirical formula 9 CHEMISTRY YEAR 11 2012 calculations using percentage composition, mass composition and combustion data
investigate a biological, environmental or industrial process applicable to the context/s chosen e.g. catalytic cracking of hydrocarbons, electroplating, electrowinning or chemistry in agriculture. Include: o a description of the chosen process o an explanation of the relationships between TASK 9 the chosen process and chemical models and theories Test o where appropriate: . safe handling and disposal of any materials or specific chemicals involved in the process . discussion of sustainability of the process . discussion of the environmental impact of the process.
TEST 6 33 ORGANIC CHEMISTRY TASK 11 Practical Reports
(Due on REVISION completion of each 34 experiment) TASK 10
END OF YEAR EXAM 2A/B
TEST 6 ORGANIC CHEMISTRY