Electronic Supplementary Material
Simultaneous determination of formaldehyde and hydrogen sulfide in air using the
cataluminescence of nanosized Zn3SnLa2O8
Kaowen Zhou1,2* Huizhen Fan1, Chunxiu Gu1,2, Baining Liu1,2,
1 Biochemical Engineering College, Beijing Union University, Beijing 100023, China 2 Biomass Waste Recycling Use of the Key Laboratory of Beijing, Beijing 100023, China
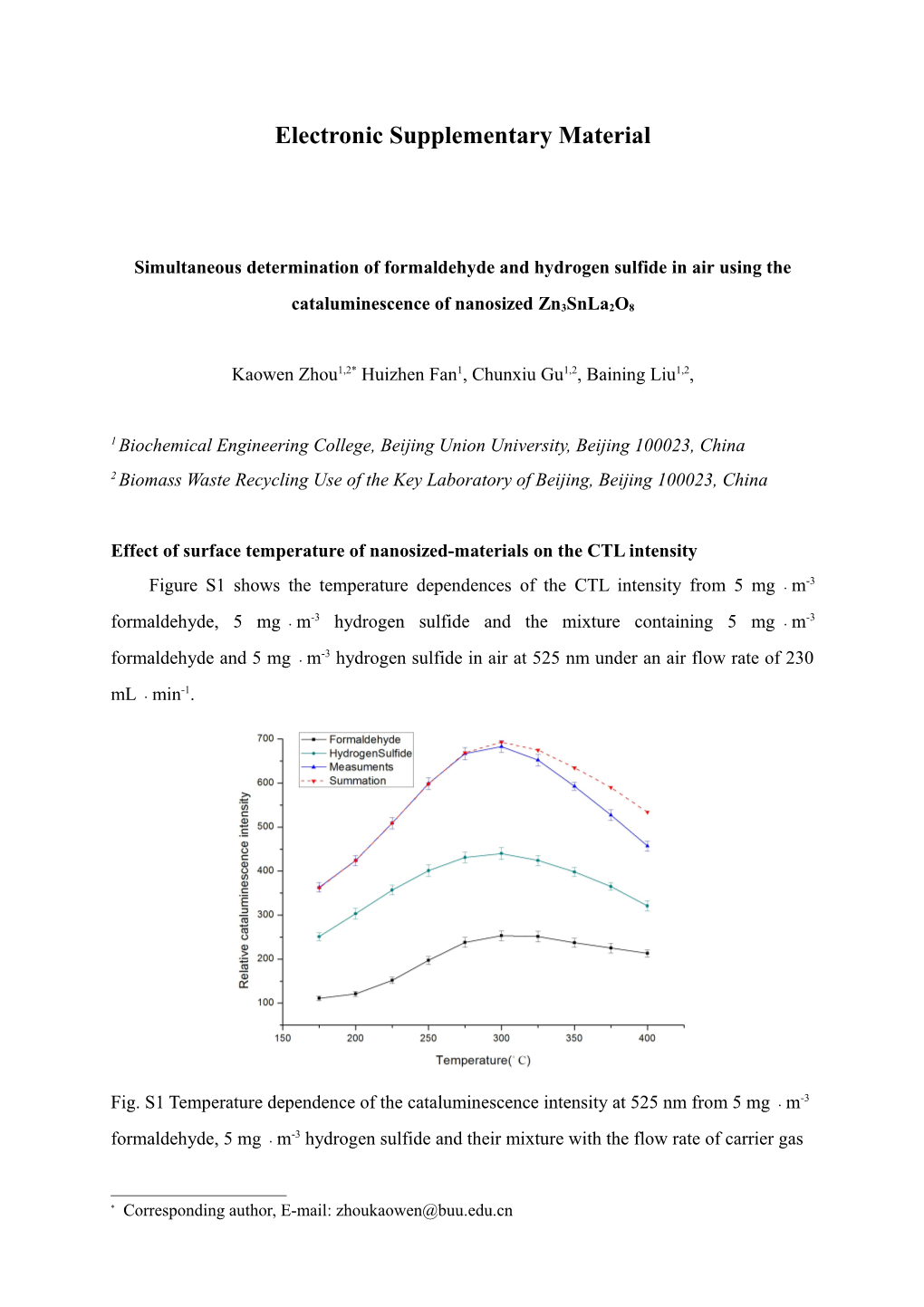
Effect of surface temperature of nanosized-materials on the CTL intensity Figure S1 shows the temperature dependences of the CTL intensity from 5 mg﹒m-3 formaldehyde, 5 mg﹒m-3 hydrogen sulfide and the mixture containing 5 mg﹒m-3 formaldehyde and 5 mg﹒m-3 hydrogen sulfide in air at 525 nm under an air flow rate of 230 mL﹒min-1.
Fig. S1 Temperature dependence of the cataluminescence intensity at 525 nm from 5 mg﹒m-3 formaldehyde, 5 mg﹒m-3 hydrogen sulfide and their mixture with the flow rate of carrier gas
* Corresponding author, E-mail: [email protected] -1 of 230 mL﹒min on nano- Zn3SnLa2O8 at the working temperature of 275 °C. As shown in Fig. S1, the luminescent signals were much larger at 300 °C from hydrogen sulfide and at 300-325 °C from formaldehyde than other temperatures at 525 nm. The measurements of the mixed vapor of formaldehyde and hydrogen sulfide, however, were not consistent with the algebraic summation of formaldehyde and hydrogen sulfide at higher temperatures than 275 °C. This means that the observation at the temperature no higher than 275 °C is preferable for the determination at 525 nm because the algebraic sum rule is applicable to the CTL intensities of formaldehyde and hydrogen sulfide in air. Fig. S2 shows the temperature dependences of the CTL intensity from 5 mg﹒m-3 formaldehyde, 5 mg﹒m-3 hydrogen sulfide and the mixture containing 5 mg﹒m-3 formaldehyde and 5 mg﹒m-3 hydrogen sulfide in air at 650 nm under an air flow rate of 230 mL﹒min-1.
Fig. S2 Temperature dependence of the cataluminescence intensity at 650 nm from 5 mg﹒m-3 formaldehyde, 5 mg﹒m-3 hydrogen sulfide and their mixture with the flow rate of carrier gas
-1 of 230 mL﹒min on nano- Zn3SnLa2O8 at the working temperature of 275 °C.
As shown in Fig. S2, the luminescent signals were much larger at 275-300 °C from formaldehyde and hydrogen sulfide than other temperatures at 650 nm, and the measurements of the mixed vapor of formaldehyde and hydrogen sulfide were almost consistent with the algebraic summation of formaldehyde and hydrogen sulfide at temperatures no higher than 300 °C. This means that the observation at 650 nm under the temperature no higher than 300 °C is preferable for the determination because the algebraic sum rule is also applicable to the CTL intensities of formaldehyde and hydrogen sulfide in air. From the above, the algebraic sum rule is applicable to the CTL intensities of formaldehyde and hydrogen sulfide in air at both 525 nm and 650 nm when the surface temperature of nanosized-materials no higher than 275 °C. The temperature of 275 °C, therefore, was chosen in following experiments.
Effect of flow rate of carrier gas Fig. S3 shows the dependences of the CTL intensity from 1 mg﹒m-3 formaldehyde at 650 nm and 1 mg﹒m-3 hydrogen sulfide at 525 nm at working temperature of 275 °C. The CTL intensity from formaldehyde increased with an increase in the flow rate below 200 mL﹒min-1, and it saturated above this flow rate. The CTL signals were strong and steady at higher flow rates. The CTL intensity from hydrogen sulfide saturated and steadied above 230 mL﹒min-1. They showed that the catalytic oxidation processes which produces the luminescent intermediates are under a diffusion controlled condition at low flow rates and are under a reaction controlled condition at high flow rates for both formaldehyde and hydrogen sulfide
[4]. A flow rate of 230 mL⋅min‾¹ was chosen because strong and steady CTL signals is obtained from both formaldehyde and hydrogen sulfide. Fig. S3 Effect of flow rate on cataluminescence intensity from 1 mg﹒m-3 formaldehyde at 650 nm and 1 mg﹒m-3 hydrogen sulfide at 525 nm at working temperature of 275 °C.
Table S1 Analysis results of formaldehyde and hydrogen sulfide in air samples with the flow
-1 rate of carrier gas of 230 mL﹒min on nano- Zn3SnLa2O8 at the working temperature of 275 °C
Sample Actual concentration Testing concentration Recovery number (mg﹒ m-3) (mg﹒ m-3) (%) Formaldehyde Hydrogen sulfide Formaldehyde Hydrogen sulfide Formaldehyde Hydrogen sulfide 1 5.00 15.00 5.13 14.70 102.6 98.0 2 10.00 10.00 9.82 9.95 98.2 99.5 3 15.00 25.00 14.79 25.68 98.6 102.7 4 20.00 20.00 20.28 19.76 101.4 98.8 5 25.00 35.00 24.30 35.46 97.2 101.3 6 30.00 30.00 30.63 30.24 102.1 100.8 7 35.00 45.00 35.28 45.49 100.8 101.1 8 40.00 40.00 39.72 39.89 99.3 99.7 9 45.00 55.00 44.51 55.48 98.9 100.9 10 50.00 50.00 49.61 49.35 99.2 98.7 11 55.00 65.00 55.50 65.78 100.9 101.2 12 60.00 60.00 60.58 59.71 101.0 99.5