Grade 11U Chemistry
Unit 1: Matter, Chemical Trends & Chemical Bonding
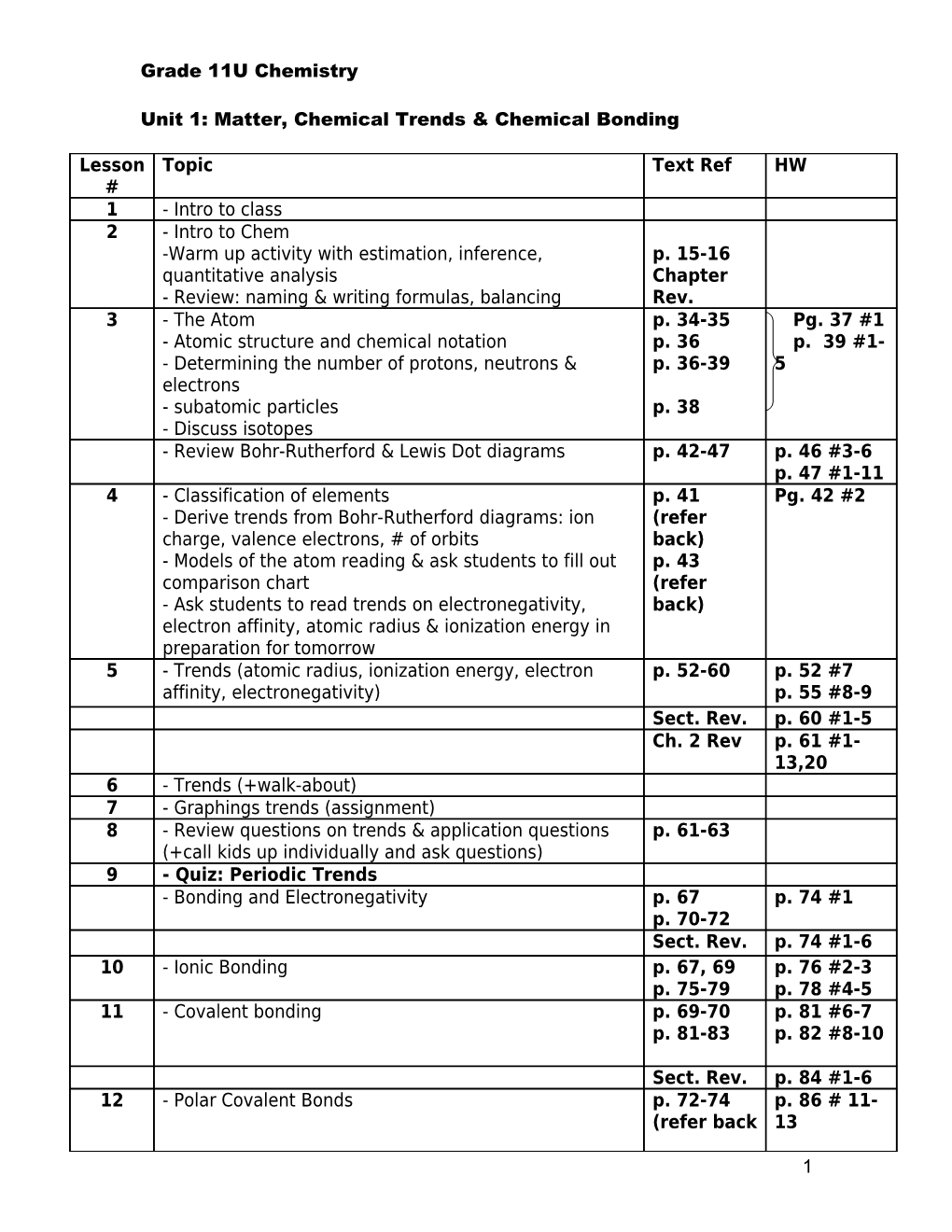
Lesson Topic Text Ref HW # 1 - Intro to class 2 - Intro to Chem -Warm up activity with estimation, inference, p. 15-16 quantitative analysis Chapter - Review: naming & writing formulas, balancing Rev. 3 - The Atom p. 34-35 Pg. 37 #1 - Atomic structure and chemical notation p. 36 p. 39 #1- - Determining the number of protons, neutrons & p. 36-39 5 electrons - subatomic particles p. 38 - Discuss isotopes - Review Bohr-Rutherford & Lewis Dot diagrams p. 42-47 p. 46 #3-6 p. 47 #1-11 4 - Classification of elements p. 41 Pg. 42 #2 - Derive trends from Bohr-Rutherford diagrams: ion (refer charge, valence electrons, # of orbits back) - Models of the atom reading & ask students to fill out p. 43 comparison chart (refer - Ask students to read trends on electronegativity, back) electron affinity, atomic radius & ionization energy in preparation for tomorrow 5 - Trends (atomic radius, ionization energy, electron p. 52-60 p. 52 #7 affinity, electronegativity) p. 55 #8-9 Sect. Rev. p. 60 #1-5 Ch. 2 Rev p. 61 #1- 13,20 6 - Trends (+walk-about) 7 - Graphings trends (assignment) 8 - Review questions on trends & application questions p. 61-63 (+call kids up individually and ask questions) 9 - Quiz: Periodic Trends - Bonding and Electronegativity p. 67 p. 74 #1 p. 70-72 Sect. Rev. p. 74 #1-6 10 - Ionic Bonding p. 67, 69 p. 76 #2-3 p. 75-79 p. 78 #4-5 11 - Covalent bonding p. 69-70 p. 81 #6-7 p. 81-83 p. 82 #8-10
Sect. Rev. p. 84 #1-6 12 - Polar Covalent Bonds p. 72-74 p. 86 # 11- (refer back 13
1 Grade 11U Chemistry
to) p. 85-88
13 - Molecular Shapes p. 88-93 p. 94 # 1-8 - Polar & Non-Polar molecules - Intermolecular forces p. 83 14 - Model building activity p. 92 15 - Bonding review 16 - Writing formulas and naming binary ionic, multivalent p. 95-97 p. 97 #14- metals & molecular compounds p. 105 15 p. 99 #16- 17 p. 105 #24 17 - Writing formulas and naming oxyanions,hydrides, p. 97-103 p. 100 #18- hydrates, polyatomic compounds & acids 19 p. 103 #20- 21 p. 104 #22 p. 105 #23 Sect. Rev. p. 106 #1-5 18 - In-class assessment of naming/writing formulas p. 154-155 Select. - Review of unit one Problems. 19 - Unit 1 Test: The Atom, Trends, Bonding, Nomenclature
2 Grade 11U Chemistry
Unit 2: Chemical Reactions
Lesson Topic Text Ref HW # 1 - Chemical Equations (skeletal, word, balanced) p. 114-118 p. 112 #1- - Balancing Chemical Equations 2 p. 113 #3- 4 p. 116 #5- 6 p. 117 #7- 8 p. 118 #9 Sect. Rev. p. 118 #1- 5 2 - Identifying types of chemical reactions lab (review) 3 - Synthesis & decomposition reactions & demos p. 119-123 p. 122 #10-13 p. 123 #14-16 p. 125 #1- 3 4 - Combustion reactions p. 123-125 p. 124 #17-20 p. 125 #4- 6 - Combustion lab/STSE Assignment 5 - Single displacement reactions & demos p. 126-127 p. 127 #21 6 - Activity series lab p. 128-129 - Activity Series p. 130-131 p. 131 - Introduce reactions assignment #22-24 7 - Double displacement reactions & demos p. 132-135 p. 134 #25 p. 135 #26-28 Sect. Rev. p. 140- 141 #1-6 8 - Copper cycle lab ( ref. youtube video) p. 138-139 9 - Review of reactions (one group per type of reaction) & each group includes… - Chapter Review p. 149-151 Select. - Unit Review p. 154-155 Problems. 10 - Unit 2 Test : Chemical Reactions
3 Grade 11U Chemistry
4 Grade 11U Chemistry
Unit 3: Chemical Quantities
Lesson Topic Text Ref HW # 1 - Significant figures, accuracy, precision p. 17-22 p. 18 #1-2 p. 22 #3 Sect. p. 24 #1-6 Rev. Pg. 29 #1- 13 - Scientific notation p. 660- p. 661 # 1-2 661 2 - Isotopes & average atomic mass p. 162- p. 167 #1-4 170 p. 169 #5-8 Sect. p. 170 #1-4 Rev. 3 - The Avogadro’s Constant and the Mole p. 171- p. 174-175 # 175 9-12 - Moles # Particles p. 175- p. 177 #13- 177 18 - #Particles Moles p. 177- p. 178 #19- 178 22 Sect. p. 179 #1-10 Rev. 4 - Molar Mass p. 180- p. 184 #23- 184 26 - Moles Mass p. 184- p. 186 #27- 186 30 - Mass Moles p. 186- p. 187 #31- 187 34 5 - Calculations involving mole concept p. 189- p. 190 #35- 192 38 p. 190 #39- 42 Sect. p. 192 #1-6 Rev. p. 193-195 Ch. Rev. 6 - Quiz - Percentage Composition: p. 198- . Law of Definite Proportions 206 . % Composition of a compound p. 198- p. 201 #1-4 199 p. 204 #5-8 p. 200- 294 Sect. p. 205 #1-8 Rev.
5 Grade 11U Chemistry
7 - Empirical Formula of a Compound p. 207- p. 209 #9-12 214 p. 211 #13- 16 Sect. p. 214 #1-8 Rev. 8 - Determining the Empirical Formula of Magnesium p. 212- Oxide Lab 213 9 - Molecular Formula of a Compound p. 215- p. 218 #17- 218 20 Sect. p. 218 #1-5 Rev. 10 - Finding Empirical & Molecular Formulas by p. 219- p. 221 #21- Experiment 222 22 - Hydrated Ionic Compounds p. 223- p. 225 # 23- 228 25 Sect. p. 228 #2, 6- Rev. 7 Ch. Rev. p. 229-231 (select. Probl.) 11 - Introduce Stoichiometry (part 1) p. 223- p. 237 #1-3 240 p. 238 #4-7 p. 240 # 8- 10 12 - Finish Stoichiometry (part 2) p. 241- p. 244 #11- 250 14 p. 246 #15- 18 p. 248 #19- 22 Sect. p.249-250 Rev. #1-7 13 - Limiting reactant p. 251- p. 254 #23- 259 26 p. 256 #27- 29 Sect. p. 269 #38- Rev. 40 14 - Percentage Yield p. 260- p. 262 #31- 270 33 p. 264 # 34- 37 (Percentage Purity) Sect. p. 270 #1-3 Rev. 15 - Quiz: Stoichiometry - percentage yield lab p. 266- 267 6 Grade 11U Chemistry
16 - Unit 3 Review p. 271- 273 17 - Unit 3 Test: Chemical Quantities
7 Grade 11U Chemistry
Unit 4: Solutions & Solubility
Lesson Topic Text Ref HW # 1 - Review of solutions p. 284-288 p. 289 #1-13 2 - Factors that affect rate of dissolving & solubility p. 290-301 - section review Sect. Rev. p. 301 #1-8 3 - Concentration of solutions Mass/Volume p. 302-305 p. 305 #1-4 Mass/Mass p. 306-308 p. 308 #5-9 Volume/Volume p. 309-310 p. 310 #10- 14 ppm/ppb p. 310-312 p. 312 #15- 18 Molar concentration p. 313-316 p. 316 #19- 24 Sect. Rev. p. 318 #1-3 4 - Solubility Curve lab p. 296-297 5 - Making solutions p. 321 #25- - Diluting solutions p. 319-324 27 p. 324 #1-5 6 - Quiz: Concentration - Predicting Solubility p. 330-335 p. 335 #1-3 p. 339 #4 - Solubility Mini Lab p. 332-333 Sect. Rev. p. 336 #1-6 7 - Total & net ionic equations p. 341-343 p. 343 #5-6 - Qualitative analysis p. 344-347 - Group activity Sect. Rev. p. 347 #1-7 8-9 - Stoichiometry in solutions p. 348-356 p. 352 #7-10 p. 355 #11- 13 Sect. Rev p. 356 #1-7 10 - Aqueous Solutions & Water Quality p. 357-364 Sect. Rev. p. 364 #1-6 11 - Quiz: Stoichiometry - Acid/Base Theories p. 370-380 p. 378 #1-3 p. 404 #1-2 Sect. Rev. p. 379 #1-10 12 - Strong/Weak Acids/Bases p. 381-393 p. 384 #4-5 p. 389 #6-9 Sect. Rev. p. 393 #1-10
8 Grade 11U Chemistry
13 - (Acid Base Reactions) p. 396-398 p. 398 # 10- - Acid-Base Titration p. 399-404 13 - Titration Set-Up step-by step - Titration Videos - Titration Calculations - Pre-lab for tomorrow’s titration lab
Sect. Rev. p. 404 #1-7 14 - Titration Lab p. 402-403 15 - Review for test p. 365-367 p. 410-411 16 - Unit 4 Test : Solutions & Solubility 17 - Water Quality/Testing Assignment 18 - Water Quality/Testing Assignment 19 - Water Quality/Testing Assignment
9 Grade 11U Chemistry
Unit 5: Gases
Lesson Topic Text Ref HW # 1 - Anticipation Guide: Gases - States of Matter p. 418-421
- Kinetic Molecular Theory of Gases p. 421-422 p. 423 #1- 7 - Introduce assignment p 2 - Pressure p. 424-428 . p. 434 #1- - Relationship between pressure & volume p. 428-4354 4 2p - Dry lab: Boyle’s law apparatus p. 430-4313. p. 435 #1-6 - Boyle’s Law p. 432-4334 - Introduce Gizmo on Boyle’s & Charles’ Law 2 3 (power point) 3 - Temperature & Volume p. 436 - Kelvin scale & absolute zero (worksheet) p. 440 p. 446 # 5-6 - Charles’ Law p.440-446 p. 449 #8-12 - Gay-Lussac’s Law p. 447-450 p. 450 #13- - Gas law demos 16 Sec. rev. p. 451 p.#1- 5 4 - Combined gas law p. 452-457 p. 457 # 17- 21 - Dalton’s law of partial pressures p. 459-458 p. 460 #22- 25 - Gas applications (read on own) p. 458, p. 462-466 Sec. rev p. 461 #1- 2,3-5 5 - Molar volume p. 472-483 p. 477 # 1-4 - Ideal gas law p. 484-488 p. 482 #5-11 p. 487 #12- 15 Sec. rev. p. 488 #1-7 6 - Applications of ideal gas law p. 489 - Molar mass and Density of unknown gas p. 490-500 p.493 #16- 19 p. 500 #20- 24 Sec. Rev. p. 500 #1-5 7 - Gas law Stoichiometry p. 501-514 p. 503 #25- 29 p. 506 #29- 34 p. 511 #35- 10 Grade 11U Chemistry
39
Sec. Rev. p. 514 #1-9 8 - Introduce AQHI Assignment - Atmospheric Reactions & Pollution p. 515-520 p. 520 #1-4 9-11 - assignment 12 - Review for test p. 521-523 13 - Unit Test
11