Head and Neck Squamous Cell Cancer and the Human Papillomavirus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reference Sheet 1
MALE SEXUAL SYSTEM 8 7 8 OJ 7 .£l"00\.....• ;:; ::>0\~ <Il '"~IQ)I"->. ~cru::>s ~ 6 5 bladder penis prostate gland 4 scrotum seminal vesicle testicle urethra vas deferens FEMALE SEXUAL SYSTEM 2 1 8 " \ 5 ... - ... j 4 labia \ ""\ bladderFallopian"k. "'"f"";".'''¥'&.tube\'WIT / I cervixt r r' \ \ clitorisurethrauterus 7 \ ~~ ;~f4f~ ~:iJ 3 ovaryvagina / ~ 2 / \ \\"- 9 6 adapted from F.L.A.S.H. Reproductive System Reference Sheet 3: GLOSSARY Anus – The opening in the buttocks from which bowel movements come when a person goes to the bathroom. It is part of the digestive system; it gets rid of body wastes. Buttocks – The medical word for a person’s “bottom” or “rear end.” Cervix – The opening of the uterus into the vagina. Circumcision – An operation to remove the foreskin from the penis. Cowper’s Glands – Glands on either side of the urethra that make a discharge which lines the urethra when a man gets an erection, making it less acid-like to protect the sperm. Clitoris – The part of the female genitals that’s full of nerves and becomes erect. It has a glans and a shaft like the penis, but only its glans is on the out side of the body, and it’s much smaller. Discharge – Liquid. Urine and semen are kinds of discharge, but the word is usually used to describe either the normal wetness of the vagina or the abnormal wetness that may come from an infection in the penis or vagina. Duct – Tube, the fallopian tubes may be called oviducts, because they are the path for an ovum. -
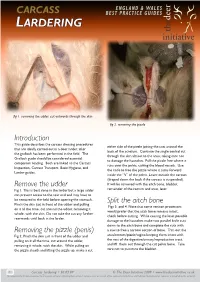
Introduction Remove the Udder Removing the Pizzle (Penis)
fig . removing the udder, cut outwards through the skin fig 2. removing the pizzle Introduction This guide describes the carcass dressing procedures either side of the pizzle joining the cuts around the that are ideally carried out in a deer larder, after back of the scrotum. Continue the single central cut the gralloch has been performed in the field. The through the skin almost to the anus, taking care not Gralloch guide should be considered essential to damage the haunches. Pull the pizzle free where it companion reading. Both are linked to the Carcass runs over the pelvis, cutting the blood vessels. Use Inspection, Carcass Transport, Basic Hygiene, and the knife to free the pizzle where it turns forward Larder guides. inside the “V” of the pelvis. Leave outside the carcass (draped down the back if the carcass is suspended). Remove the udder It will be removed with the aitch bone, bladder, Fig 1. This is best done in the larder but a large udder remainder of the rectum and anus, later. can prevent access to the rear end and may have to be removed in the field before opening the stomach. Split the aitch bone Pinch the skin just in front of the udder and pulling Figs 3. and 4. Note that some venison processors on it all the time, cut around the udder, removing it would prefer that the aitch bone remains intact, whole, with the skin. Do not take the cut any further check before cutting. While causing the least possible rearwards until back in the larder. -

Preventive Health Care
PREVENTIVE HEALTH CARE DANA BARTLETT, BSN, MSN, MA, CSPI Dana Bartlett is a professional nurse and author. His clinical experience includes 16 years of ICU and ER experience and over 20 years of as a poison control center information specialist. Dana has published numerous CE and journal articles, written NCLEX material, written textbook chapters, and done editing and reviewing for publishers such as Elsevire, Lippincott, and Thieme. He has written widely on the subject of toxicology and was recently named a contributing editor, toxicology section, for Critical Care Nurse journal. He is currently employed at the Connecticut Poison Control Center and is actively involved in lecturing and mentoring nurses, emergency medical residents and pharmacy students. ABSTRACT Screening is an effective method for detecting and preventing acute and chronic diseases. In the United States healthcare tends to be provided after someone has become unwell and medical attention is sought. Poor health habits play a large part in the pathogenesis and progression of many common, chronic diseases. Conversely, healthy habits are very effective at preventing many diseases. The common causes of chronic disease and prevention are discussed with a primary focus on the role of health professionals to provide preventive healthcare and to educate patients to recognize risk factors and to avoid a chronic disease. nursece4less.com nursece4less.com nursece4less.com nursece4less.com 1 Policy Statement This activity has been planned and implemented in accordance with the policies of NurseCe4Less.com and the continuing nursing education requirements of the American Nurses Credentialing Center's Commission on Accreditation for registered nurses. It is the policy of NurseCe4Less.com to ensure objectivity, transparency, and best practice in clinical education for all continuing nursing education (CNE) activities. -

Clinical Implications of HPV in Head and Neck Cancers
VOLUME 24 ⅐ NUMBER 17 ⅐ JUNE 10 2006 JOURNAL OF CLINICAL ONCOLOGY REVIEW ARTICLE Clinical Implications of Human Papillomavirus in Head and Neck Cancers Carole Fakhry and Maura L. Gillison From the Departments of Viral Oncol- ABSTRACT ogy, Aerodigestive Malignancy, and Cancer Prevention and Control, The Human papillomavirus (HPV) is now recognized to play a role in the pathogenesis of a subset of head and neck Sidney Kimmel Comprehensive Cancer squamous cell carcinomas (HNSCCs), particularly those that arise from the lingual and palatine tonsils within Center at Johns Hopkins; and the the oropharynx. High-risk HPV16 is identified in the overwhelming majority of HPV-positive tumors, which have Departments of Epidemiology and molecular-genetic alterations indicative of viral oncogene function. Measures of HPV exposure, including Molecular Microbiology and Immunol- sexual behaviors, seropositivity to HPV16, and oral, high-risk HPV infection, are associated with increased risk ogy, The Johns Hopkins Bloomberg for oropharyngeal cancer. HPV infection may be altering the demographics of HNSCC patients, as these School of Public Health, Baltimore, MD. patients tend to be younger, nonsmokers, and nondrinkers. There is sufficient evidence to conclude that a Submitted February 9, 2006; accepted diagnosis of HPV-positive HNSCC has significant prognostic implications; these patients have at least half the February 27, 2006. risk of death from HNSCC when compared with the HPV-negative patient. The HPV etiology of these tumors Supported in part by National Institute may have future clinical implications for the diagnosis, therapy, screening, and prevention of HNSCC. for Dental and Craniofacial Research Grant No. DE016631-02. J Clin Oncol 24:2606-2611. -

50 Facts About Oral Head and Neck Cancer
50 Facts about Oral, Head and Neck Cancer 1. Oral, Head and Neck Cancer most commonly refers to squamous cell carcinoma of the tongue, throat, and voice box. However, often, head and neck cancer also refers to other types of cancer that arises in the nasal cavity, sinuses, lips, mouth, thyroid glands, salivary glands, throat, or voice box. 2. HPV or human papillomavirus appears to be responsible for the rise in cancers of the oropharynx (tonsil and base of tongue) in younger nonsmokers and is related to oral sex. 3. Tobacco and alcohol use are the leading causes of mouth and voice box cancers. 4. Cancers of the head and neck account for 6 percent of all malignancies in the United States. 5. Caucasians currently have the highest incidence rates of head and neck cancers, although death is still highest in African Americans. 6. Tobacco (including smokeless tobacco) and alcohol use are very important risk factors for oral, head and neck cancers, particularly those of the tongue, mouth, throat and voice box. Chewing tobacco has been shown to cause mouth cancer. Human Papilloma Virus may be related to over half of tonsil cancers. 7. Cigarette smoking increases your risk of head and neck cancer by 15 times compared to a non-smoker. 8. People who use both tobacco and alcohol are at greater risk than people who use them alone. 9. Oral, Head and Neck cancers tend to form in the areas where tobacco/alcohol use has the most contact. For example, where the cigarette sits on the lip, or where the chewing tobacco is placed in the mouth. -

Primary Screening for Breast Cancer with Conventional Mammography: Clinical Summary
Primary Screening for Breast Cancer With Conventional Mammography: Clinical Summary Population Women aged 40 to 49 y Women aged 50 to 74 y Women aged ≥75 y The decision to start screening should be No recommendation. Recommendation Screen every 2 years. an individual one. Grade: I statement Grade: B Grade: C (insufficient evidence) These recommendations apply to asymptomatic women aged ≥40 y who do not have preexisting breast cancer or a previously diagnosed high-risk breast lesion and who are not at high risk for breast cancer because of a known underlying genetic mutation Risk Assessment (such as a BRCA1 or BRCA2 gene mutation or other familial breast cancer syndrome) or a history of chest radiation at a young age. Increasing age is the most important risk factor for most women. Conventional digital mammography has essentially replaced film mammography as the primary method for breast cancer screening Screening Tests in the United States. Conventional digital screening mammography has about the same diagnostic accuracy as film overall, although digital screening seems to have comparatively higher sensitivity but the same or lower specificity in women age <50 y. For women who are at average risk for breast cancer, most of the benefit of mammography results from biennial screening during Starting and ages 50 to 74 y. While screening mammography in women aged 40 to 49 y may reduce the risk for breast cancer death, the Stopping Ages number of deaths averted is smaller than that in older women and the number of false-positive results and unnecessary biopsies is larger. The balance of benefits and harms is likely to improve as women move from their early to late 40s. -

View of Urothelial and Metastatic Carcinoma Including Clinical Presentation, Diagnostic Testing, Treatment and Chiropractic Considerations Is Discussed
Daniels et al. Chiropractic & Manual Therapies (2016) 24:14 DOI 10.1186/s12998-016-0097-8 CASE REPORT Open Access Bladder metastasis presenting as neck, arm and thorax pain: a case report Clinton J. Daniels1,2,3*, Pamela J. Wakefield1,2 and Glenn A. Bub1,2 Abstract Background: A case of metastatic carcinoma secondary to urothelial carcinoma presenting as musculoskeletal pain is reported. A brief review of urothelial and metastatic carcinoma including clinical presentation, diagnostic testing, treatment and chiropractic considerations is discussed. Case presentation: This patient presented in November 2014 with progressive neck, thorax and upper extremity pain. Computed tomography revealed a destructive soft tissue mass in the cervical spine and additional lytic lesion of the 1st rib. Prompt referral was made for surgical consultation and medical management. Conclusion: Distant metastasis is rare, but can present as a musculoskeletal complaint. History of carcinoma should alert the treating chiropractic physician to potential for serious disease processes. Keywords: Chiropractic, Neck pain, Transitional cell carcinoma, Bladder cancer, Metastasis, Case report Background serious complication of UC is distant metastasis—with Urothelial carcinoma (UC), also known as transitional higher stage cancer and lymph involvement worsening cell carcinoma (TCC), accounts for more than 90 % of prognosis and cancer survival rate [10]. The 5-year all bladder cancers and commonly metastasizes to the cancer-specific survival rate of UC is estimated to be pelvic lymph nodes, lungs, liver, bones and adrenals or 78 % [10, 11]. brain [1, 2]. The spread of bladder cancer is mainly done Neck pain accounts for 24 % of all disorders seen by via the lymphatic system with the most frequent location chiropractors [12]. -

The Contagious Head and Neck Cancer
omens H f W ea o l l th a n C r a u r e o J Hoffman-Ruddy, et al., J Women’s Health Care 2015, 4:2 Journal of Women's Health Care DOI: 10.4172/2167-0420.1000226 ISSN: 2167-0420 Review Article Open Access The Contagious Head and Neck Cancer: The Role of Human Papillomavirus HPV Bari Hoffman-Ruddy*1, Sarah Miller2, Erin Silverman3, Vicki Lewis4, Henry Ho4 and Christine Sapienza5 1Department of Communication Sciences and Disorders, University of Central Florida, College of Health and Public Affairs, Florida, USA 2University of Memphis, Loewenberg School of Nursing 3Department of Physiology, University of Florida, College of Veterinary Medicine, Florida, USA 4Florida Hospital Cancer Institute, The Ear Nose Throat and Plastic Surgery Associates 5Department of Communication Sciences and Disorders, College of Health Sciences, Brooks Rehabilitation, Jacksonville University *Corresponding author: Bari Hoffman-Ruddy, University of Central Florida, College of Health and Public Affairs, Department of Communication Sciences and Disorders, Florida, USA, Tel: 407-823-4894; Fax: 407-823-4816; E-mail: [email protected] Received date: Jan 06, 2015; Accepted date: Feb 20, 2015; Published date: Feb 25, 2015 Copyright: © 2015 Hoffman-Ruddy B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Keywords: Human papillomavirus, Head and neck cancer, have no history of significant tobacco or alcohol use [8]. In fact, rates Transmitted diseases of certain cancers of the oropharynx have increased over the last 30 years among young adults who have never smoked or used tobacco Summary products [9]. -

Understanding the Randomized Controlled Trials By
Breast Cancer Screening: Understanding the Randomized Controlled Trials By: Phoebe Freer, MD, Linda Moy, MD, FSBI, Wendy DeMartini, MD, FSBI, and the Screening Leadership Group Screening mammography has been shown to decrease breast cancer mortality across multiple trials, and across many different study designs. Despite this, some opponents continue to question the value of mammography. Thus, it is increasingly important that breast imaging care providers understand the nature and results of the randomized controlled trials (RCTs), which have definitively demonstrated that screening mammography in women 40-74 years of age decreases deaths from breast cancer. Cancer localized in the breast is not what causes death; it is breast cancer spread (metastasis) to other organs that causes mortality. The goal of mammographic screening (and other breast cancer screening tests) is to detect breast cancer earlier than it would otherwise manifest clinically, when it is less likely to have spread. Data clearly show that detection of breast cancers at smaller sizes and lower stages is associated with better patient outcomes from lower morbidity and reduced breast cancer deaths. RCTs are the gold standard for proving that early detection with mammography decreases mortality from breast cancer. It is important to understand that the key evidence measure is the breast cancer death rate observed in the experimental group (women invited to have screening mammography) compared to that in the control group (women not invited to have screening mammography). It is not sufficient to use survival time (the time of discovery of the cancer to the date of death) between the groups, as this may reflect “lead-time” bias, in which a cancer is found earlier so survival time appears longer, but the date of death is not altered. -

Clinical Radiation Oncology Review
Clinical Radiation Oncology Review Daniel M. Trifiletti University of Virginia Disclaimer: The following is meant to serve as a brief review of information in preparation for board examinations in Radiation Oncology and allow for an open-access, printable, updatable resource for trainees. Recommendations are briefly summarized, vary by institution, and there may be errors. NCCN guidelines are taken from 2014 and may be out-dated. This should be taken into consideration when reading. 1 Table of Contents 1) Pediatrics 6) Gastrointestinal a) Rhabdomyosarcoma a) Esophageal Cancer b) Ewings Sarcoma b) Gastric Cancer c) Wilms Tumor c) Pancreatic Cancer d) Neuroblastoma d) Hepatocellular Carcinoma e) Retinoblastoma e) Colorectal cancer f) Medulloblastoma f) Anal Cancer g) Epndymoma h) Germ cell, Non-Germ cell tumors, Pineal tumors 7) Genitourinary i) Craniopharyngioma a) Prostate Cancer j) Brainstem Glioma i) Low Risk Prostate Cancer & Brachytherapy ii) Intermediate/High Risk Prostate Cancer 2) Central Nervous System iii) Adjuvant/Salvage & Metastatic Prostate Cancer a) Low Grade Glioma b) Bladder Cancer b) High Grade Glioma c) Renal Cell Cancer c) Primary CNS lymphoma d) Urethral Cancer d) Meningioma e) Testicular Cancer e) Pituitary Tumor f) Penile Cancer 3) Head and Neck 8) Gynecologic a) Ocular Melanoma a) Cervical Cancer b) Nasopharyngeal Cancer b) Endometrial Cancer c) Paranasal Sinus Cancer c) Uterine Sarcoma d) Oral Cavity Cancer d) Vulvar Cancer e) Oropharyngeal Cancer e) Vaginal Cancer f) Salivary Gland Cancer f) Ovarian Cancer & Fallopian -

Laryngeal Cancer Survivorship
About the Authors Dr. Yadro Ducic MD completed medical school and Head and Neck Surgery training in Ottawa and Toronto, Canada and finished Facial Plastic and Reconstructive Surgery at the University of Minnesota. He moved to Texas in 1997 running the Department of Otolaryngology and Facial Plastic Surgery at JPS Health Network in Fort Worth, and training residents through the University of Texas Southwestern Medical Center in Dallas, Texas. He was a full Clinical Professor in the Department of Otolaryngology-Head Neck Surgery. Currently, he runs a tertiary referral practice in Dallas-Fort Worth. He is Director of the Baylor Neuroscience Skullbase Program in Fort Worth, Texas and the Director of the Center for Aesthetic Surgery. He is also the Codirector of the Methodist Face Transplant Program and the Director of the Facial Plastic and Reconstructive Surgery Fellowship in Dallas-Fort Worth sponsored by the American Academy of Facial Plastic and Reconstructive Surgery. He has authored over 160 publications, being on the forefront of clinical research in advanced head and neck cancer and skull base surgery and reconstruction. He is devoted to advancing the care of this patient population. For more information please go to www.drducic.com. Dr. Moustafa Mourad completed his surgical training in Head and Neck Surgery in New York City from the New York Eye and Ear Infirmary of Mt. Sinai. Upon completion of his training he sought out specialization in facial plastic, skull base, and reconstructive surgery at Baylor All Saints, under the mentorship and guidance of Dr. Yadranko Ducic. Currently he is based in New York City as the Division Chief of Head and Neck and Skull Base Surgery at Harlem Hospital, in addition to being the Director for the Center of Aesthetic Surgery in New York. -

Oral Cancer Fact Sheet
Want Some Life Saving Advice? Ask Your Dental Hygienist About Oral Cancer 8/11/10 This year alone, more than 30,000 Americans will be di- • Sore throats that do not go away, or a feeling that agnosed with oral cancer and 8,000 will die of the disease. some¬ thing is caught in the throat Oral cancer is more common than leukemia, Hodgkin’s • Difficulty or pain with chewing or swallowing disease and cancers of the brain, liver, bone and stom- ach, and is typically caused by long-term use of tobacco Treatment products, alcohol and human papilloma virus (HPV) infec- As researchers continually seek out more effective tion. According to the National Cancer Institute (NCI), Oral drugs and drug combinations to help combat oral cancer, cancer is a major cause of death and disfigurement in the the most common current treatment for oral cancer, ac- United States. cording to NCI, is to remove any tumors surgically. Oral cancer also may be treated using intensive Risk Factors Approximately 75% of all oral cavity and pharyngeal cancers—mouth, tongue, lips, throat, parts of the nose Oral Cancer Self-Exam and larynx—are attributed to the use of smoked and smoke¬less tobacco, according to the Centers for Disease The following is an oral cancer self-examination that can Control and Prevention (CDC). Those who choose to use be taught to patients. cigarettes, cigars, pipes, chewing tobacco, snuff and/or bidis (cigarettes from India that come in a variety of fla- Look at and feel your: vors and contain less tobacco than regular U.S.