New Indep Link Template.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Lee, Blackburn Claim TN Victory
6,250 subscribers www.TML1.org Volume 69, Number 19 Nov. 12, 2018 Lee, Blackburn claim TN victory TML District Meetings Thursday, Nov. 29 District 4 Crossville Friday, Nov. 30 District 3 Collegedale Tuesday, Dec. 4 District 5 Gallatin Wednesday, Dec. 5 District 2 Morristown Thursday, Dec. 6 District 1 Greeneville Monday, Dec. 10 District 7 Henderson Tuesday, Dec. 11 District 8 Millington Friday, Dec 14 District 6 Columbia Mark your calendars and plan to attend! Photos by The Tennessean Tennesseans elected Repub- And I couldn’t be more grateful,” Corker, who after serving two lican Bill Lee as the state’s 50th Lee said during his acceptance terms opted not to run again. governor on Nov. 6, voting into speech. “I’m grateful you placed Blackburn won the seat with office a political newcomer. Lee your trust in us to lead this great 55 percent of the votes to Bre- easily defeated former Nashville state of Tennessee.” desen’s 44 percent. Mayor Karl Dean with 60 percent Lee will be sworn into office Blackburn has served the of the votes to Dean’s 39 percent. on Saturday, Jan. 19, 2019, in 7th Congressional District in the Lee, 59, is a Tennessean busi- Nashville. House of Representatives since nessman and CEO of his family’s Republican Marsha Blackburn 2003. She previously served in the HVAC, plumbing, and electrical defeated former Tennessee Gov- Tennessee Senate from 1999 to business, Lee Company. He cam- ernor Phil Bredesen to become 2003. paigned on a socially and fiscally Tennessee’s first female elected to For a complete results of conservative platform. -

General Election State of Tennessee Tennessee House of Representatives District 1
State of Tennessee November 6, 2012 General Election Tennessee House of Representatives District 1 1 . Jon C. Lundberg - (R) 1 SULLIVAN 17,503 DISTRICT TOTALS 17,503 05-Dec-12 State of Tennessee November 6, 2012 General Election Tennessee House of Representatives District 2 1 . Tony Shipley - (R) 2 . Bruce Dotson - (D) 1 2 SULLIVAN 16,764 7,794 DISTRICT TOTALS 16,764 7,794 05-Dec-12 State of Tennessee November 6, 2012 General Election Tennessee House of Representatives District 3 1 . Timothy Hill - (R) 2 . Leah R. Kirk - (D) 3 . Suzanne Parker - (G) 1 2 3 CARTER 2,747 521 148 JOHNSON 4,300 1,018 238 SULLIVAN 8,963 2,880 449 DISTRICT TOTALS 16,010 4,419 835 05-Dec-12 State of Tennessee November 6, 2012 General Election Tennessee House of Representatives District 4 1 . Thomas Gray - (R) 2 . Kent Williams - (I) 1 2 CARTER 6,334 9,112 UNICOI 3,553 2,361 DISTRICT TOTALS 9,887 11,473 05-Dec-12 State of Tennessee November 6, 2012 General Election Tennessee House of Representatives District 5 1 . David B. Hawk - (R) 2 . Eddie Yokley - (D) 3 . Write-In - Jason Scott Moore 1 2 3 GREENE 11,566 8,292 1 DISTRICT TOTALS 11,566 8,292 1 05-Dec-12 State of Tennessee November 6, 2012 General Election Tennessee House of Representatives District 6 1 . James (Micah) Van Huss - (R) 2 . Michael Clark - (D) 1 2 WASHINGTON 16,391 6,271 DISTRICT TOTALS 16,391 6,271 05-Dec-12 State of Tennessee November 6, 2012 General Election Tennessee House of Representatives District 7 1 . -

Legislators' Positions Compared to Chamber Positions
Legislators' Positions Compared to Chamber Positions Positions of legislators based on voting record and bill sponsors. Right-to-Work Safe Harbor Pregnant Workers Criminal Senator Tobacco Sales Safe Harbor Transpotainment Amendment Conference Report Fairness Act Justice Reform Steve Dickerson (R-20) NVR √ NVR √ √ √ √ Brenda Gilmore (D-19) X √ X X √ √ √ Ferrell Haile (R-18) √ √ √ √ √ √ Joey Hensley (R-28) √ √ √ NVR √ √ Jack Johnson (R-23) √ √ √ √ √ √ Mark Pody (R-17) NVR X √ √ √ √ √ Bill Powers (R-22) √ √ √ √ √ √ Shane Reeves (R-14) √ √ √ √ √ √ Kerry Roberts (R-25) √ PNV √ √ √ √ Dawn White (R-13) √ √ √ √ √ √ Jeff Yarbro (D-21) NVR √ X X √ √ Lt. Governor Randy McNally (R-5) √ √ √ √ √ √ Blank spaces indicate that the legislator did not have an opportunity to vote on the bill. BOLD TEXT Davidson County Delegation UN-BOLD Middle Tennessee delegation √ Supported Chamber position X Opposed to Chamber position PNV Present but not voting NVR No vote recorded (absent) Sponsored/Co-sponsored Chamber-endorsed bill Sponsored/Co-sponsored Chamber-opposed bill ` Legislators' Positions Compared to Chamber Positions Positions of legislators based on voting record and bill sponsors. Right-to-Work Safe Harbor Pregnant Workers Criminal Justice Representative Tobacco Sales Safe Harbor Amendment Conference Report Fairness Act Reform Charie Baum (R-37) √ √ √ √ √ √ Bill Beck (D-51) X √ X X √ √ Clark Boyd (R-46) √ X √ √ √ √ Glen Casada (R-62) √ √ √ √ NVR √ Scott Cepicky (R-64) √ √ √ √ √ √ John Ray Clemmons (D-55) X √ X X √ √ Michael Curcio (R-69) √ X √ X √ √ Vincent Dixie (D-54) X √ PNV X √ √ Bob Freeman (D-56) X √ √ PNV √ √ Johnny Garrett (R-45) √ PNV √ X √ √ Jason Hodges (D-67) X PNV X X √ √ Darren Jernigan (D-60) X √ √ NVR √ √ Curtis Johnson (R-68) √ √ √ X √ √ Sabi Kumar (R-66) √ √ √ NVR √ √ William Lamberth (R-44) √ √ √ X √ X Mary Littleton (R-78) √ √ √ PNV √ √ Harold Love, Jr. -

Tennessee Right to Life Scorecard – Pro-Life Legislation Votes, 2015 Tennessee House of Representatives 109Th General Assembly
PO Box 110765 Nashville, TN 37222-0765 Ph 615.298.5433 [email protected] Facebook.com/groups/tnrtl/ www.tnrtl.org Twitter.com/tnrighttolife Tennessee Right to Life Scorecard – Pro-Life Legislation Votes, 2015 Tennessee House of Representatives 109th General Assembly 1. House Floor Vote on SB1222/HB0977 (Informed Consent for Women and Girls Considering Abortion and a 48-hour Waiting Period), April 21, 2015. (Passed 79-18) 2. House Floor Vote on SB1280/HB1368 (Regulation of Abortion Facilities, requiring inspection and licensure), April 21, 2015 (Passed 80-17) Representative 1 2 X O Score Speaker Beth Harwell (R-Nashville) X X 2 0 100% Raumesh Akbari (R-Winchester) O O 0 2 0% David Alexander (R-Winchester) X X 2 0 100% Joe Armstrong (D-Knoxville) O O 0 2 0% Bill Beck (D-Nashville) A A 0 0 N/A Harry Brooks (R-Knoxville) X X 2 0 100% Kevin Brooks (R-Cleveland) X X 2 0 100% Sheila Butt (R-Columbia) X X 2 0 100% David Byrd (R – Waynesboro) X X 2 0 100% Kent Calfee (R-Kingston) X X 2 0 100% Karen Camper (D-Memphis) O O 0 2 0% Dale Carr (R-Sevierville) X X 2 0 100% Mike Carter (R-Ooltewah) X X 2 0 100% Glen Casada (R-Thompsons Station) X X 2 0 100% John Ray Clemmons (D-Nashville) O O 0 2 0% Jim Coley (R-Bartlett) X X 2 0 100% Barbara Cooper (D-Memphis) O O 0 2 0% Martin Daniel (R-Knoxville) X X 2 0 100% John DeBerry (D-Memphis) X X 2 0 100% Barry Doss (R-Leoma) X X 2 0 100% Kevin Dunlap (D-Rock Island) X X 2 0 100% Bill Dunn (R-Knoxville) X X 2 0 100% Jeremy Durham (R-Franklin) X X 2 0 100% Jimmy Eldridge (R-Jackson) X X 2 0 100% Jeremy Faison (R-Cosby) X X 2 0 100% Andrew Farmer (R-Sevierville) X X 2 0 100% Joanne Favors (D-Chattanooga) O O 0 2 0% Craig Fitzhugh (D-Ripley) O X 1 1 50% John Forgety (R-Athens) X X 2 0 100% Brenda Gilmore (D-Nashville) O O 0 2 0% Tilman Goins (R-Morristown) X X 2 0 100% Marc Gravitt (R-East Ridge) X X 2 0 100% Curtis Halford (R-Dyer) X X 2 0 100% G.A. -

Indep Link Beg 2.21.18.Indd
Independent Tennessee Pharmacists Association www.tnpharm.org • [email protected] • 615.256.3023 Link March 28, 2019 KEEP IT UP! PBM Reform Hearings on Tuesday, April 2 THANK YOU for your advocacy and engagement, includ- What actions are needed TODAY? ing emails and calls to key legislators to gain support for This legislation still faces signifi cant opposition, so your House Bill 786 and Senate Bill 650! Your voices of support continued engagement is critically important for it to be- are being heard loud and clear at the Capitol in Nashville! come law. Stand up and advocate on behalf of your pa- tients, and share the importance and value of the phar- What is the current status of the legislation? macy profession with the key legislators below. House Bill 786 and Senate Bill 650 were scheduled to be heard on March 26, but both bills were rolled a week by More about this legislation: the sponsors, in order to fi nalize language and resolve TPA has worked with Representative Cameron Sexton, any remaining fi scal note issues (costs to the state). Roll- Senator Shane Reeves, Senator Ferrell Haile, Represen- ing a bill has no bearing on the status or fate of the leg- tative Bryan Terry, and other pharmacy champion leg- islation. However, it does give you a few more days to islators, to introduce several pieces of legislation which reach out to key legislators to ask for their support. On would provide support to the pharmacy profession and Tuesday, April 2, House Bill 786 is scheduled to be heard bring increased transparency within the prescription in the House Insurance Committee and Senate Bill 650 drug delivery system. -

47 Thursday, January 13, 2011 Third Organizational
THURSDAY, JANUARY 13, 2011 THIRD ORGANIZATIONAL DAY The House met at 9:00 a.m., and was called to order by Madam Speaker Harwell. The proceedings were opened with prayer by Reverend Roderick J. Glatt, Mt. Gilead Baptist Church, Nashville, TN. Representative Gilmore led the House in the Pledge of Allegiance to the Flag. ROLL CALL The roll call was taken with the following results: Present....................................................................................... 97 Representatives present were Alexander, Armstrong, Bass, Brooks H, Brooks K, Brown, Butt, Campbell, Camper, Carr, Cobb, Coley, Cooper, Curtiss, Dean, DeBerry J, DeBerry L, Dennis, Dunn, Elam, Eldridge, Evans, Faison, Favors, Fitzhugh, Floyd, Ford, Forgety, Gilmore, Gotto, Halford, Hall, Hardaway, Harmon, Harrison, Hawk, Haynes, Hensley, Hill, Holt, Hurley, Johnson C, Johnson P, Jones S, Keisling, Kernell, Lollar, Lundberg, Maggart, Marsh, Matheny, Matlock, McCormick, McDaniel, McDonald, McManus, Miller D, Miller L, Montgomery, Moore, Naifeh, Niceley, Odom, Pitts, Pody, Powers, Pruitt, Ragan, Ramsey, Rich, Richardson, Roach, Sanderson, Sargent, Sexton, Shaw, Shepard, Shipley, Sontany, Sparks, Stewart, Swann, Tidwell, Tindell, Todd, Towns, Turner J, Turner M, Watson, Weaver, White, Williams K, Williams R, Windle, Wirgau, Womick, Madam Speaker Harwell -- 97 EXCUSED The Speaker announced that the following member(s) has/have been excused, pursuant to request(s) under Rule No. 20: Representative Casada; business reasons PERSONAL ORDERS RECOGNITION IN THE WELL Representative L. DeBerry was recognized in the Well in order to lead the memorial service, “Celebrating the Life and Legacy of the Honorable Ulysses Jones, Jr.” Representative Curtiss was recognized in the Well to lead the House in a prayer for the late Representative Ulysses Jones. -

Voter Guide Inside
TN YOUR VOTE WILL CHANGE THE DIRECTION OF THE COUNTRY. STATE HOUSE District 1 Jon Lundberg* District 63 Glen Casada Election Day is November 4, 2014 It is critical to be informed and vote for candidates that support small business. NFIB’s SAFE Trust PACs endorsed the following candidates because District 2 Bud Hulsey District 64 Sheila Butt they are willing to stand up for America’s small business owners, thereby District 3 Timothy Hill District 65 Jeremy Durham protecting the families, employees and communities that depend on them. District 5 David Hawk District 66 Sabi “Doc” Kumar District 6 Micah Van Huss District 68 Curtis Johnson* VOTER REGISTRATION DEADLINE Endorsements are current as of mailing date. District 7 Matthew Hill District 70 Barry Doss* Voter registration ends October 6. District 8 Art Swann District 71 David “Coach” Byrd District 9 Michael Harrison District 72 Steve McDaniel* EARLY & ABSENTEE VOTING District 10 Tilman Goins District 73 Jimmy Eldridge* District 11 Jeremy Faison District 75 Tim Wirgau Early voting in person is available for all registered voters. Vote early District 12 Dale Carr District 76 Andy Holt by mail is available under certain circumstances. Please contact District 13 Eddie Smith District 77 Bill Sanderson your local elections office for more information. District 14 Ryan Haynes District 78 Mary Littleton District 16 Bill Dunn District 79 Curtis Halford IMPORTANT DATES: District 17 Andrew Farmer District 81 Debra Moody Early in-person voting begins October 15 and ends October 30. District 18 Martin Daniel* District 83 Mark White* The last day to request an absentee ballot is October 28. -

List of Local/Regional Elected Officials Who Has Been Invited, Provided the Zoom Link, and Will Be Granted Access to Participate in the Meeting
“COVID & the Community – Our Next Steps Together” INVITATION LIST (revised: 12/12/20 – LJ) [[ PARTICIPANTS ]] List of local/regional elected officials who has been invited, provided the Zoom link, and will be granted access to participate in the meeting. Additional invitations may still be added. The meeting will be streamed for media and the public at: stream.knoxcountytn.gov All participants have also been asked to send their questions in advance to Senator Briggs ([email protected]) to collate and organize. STATE SENATORS • Lt. Governor Randy McNally • Sen. Richard Briggs • Sen. Becky Massey • Sen. Frank Nicely • Sen. Steve Southerland • Sen. Art Swann • Sen. Ken Yager STATE REPRESENTATIVES • Speaker Cameron Sexton • Rep. Dave Wright • Rep. Michelle Carringer • Rep. Justin Lafferty • Rep. Eddie Mannis • Rep. Gloria Johnson • Rep. Sam McKenzie • Rep. Jason Zachary • Rep. Kent Calfee • Rep. Dale Carr • Rep. Rick Eldridge • Rep. Jeremy Faison • Rep. Andrew Farmer • Rep. Kelly Keisling • Rep. Jarome Moon • Rep. Dennis Powers • Rep. John Ragan • Rep. Bob Ramsey • Rep. Lowell Russell • Rep. Jerry Sexton MAYORS • Knox Co. Mayor Glenn Jacobs • Knoxville Mayor Indya Kincannon • Farragut Mayor Ron Williams • Blount Co. Mayor Ed Mitchell • Anderson Co. Mayor Terry Frank • Sevier Co. Mayor Larry Waters • Loudon Co. Mayor Buddy Bradshaw • Roane Co. Executive Ron Woody • Union Co. Mayor Jason Bailey • Grainger Co. Mayor Mike Byrd • Jefferson Co. Mayor Mark Potts COUNTY COMMISSIONERS • Commissioner Dasha Lundy • Commissioner Courtney Durrett • Commissioner Randy Smith • Commissioner Kyle Ward • Commissioner John Schoonmaker • Commissioner Terry Hill • Commissioner Charles Busler • Commissioner Richie Beeler • Commissioner Carson Dailey • Commissioner Larsen Jay • Commissioner Justin Biggs CITY COUNCIL REPRESENTATIVES • Council Tommy Smith • Andrew Roberto • Seema Singh • Lauren Rider • Charles Thomas • Gwen McKenzie • Lynne Fugate • Janet Testerman • Amelia Parker TOWN OF FARRAGUT ALDERMEN • Lousie Povlin • Ron Pinchok • Scott Meyer • Drew Burnette . -

State of Tennessee State General United States President United
State of Tennessee November 3, 2020 State General United States President 1 Donald J. Trump - Republican 1,852,475 2 Joseph R. Biden - Democratic 1,143,711 3 Don Blankenship - Independent 5,365 4 Roque "Rocky" De La Fuente - Independent 1,860 5 Howie Hawkins - Independent 4,545 6 Jo Jorgensen - Independent 29,877 7 Alyson Kennedy - Independent 2,576 8 Gloria La Riva - Independent 2,301 9 Kanye West - Independent 10,279 10 Write-In - R19 Boddie 1 11 Write-In - Brian Carroll 762 12 Write-In - Tom Hoefling 31 13 Write-In - Jade Simmons 68 14 Write-In - Kasey Wells 0 Total Votes 3,053,851 United States Senate 1 Bill Hagerty - Republican 1,840,926 2 Marquita Bradshaw - Democratic 1,040,691 3 Yomi "Fapas" Faparusi Sr. - Independent 10,727 4 Jeffrey Alan Grunau - Independent 4,160 5 Ronnie Henley - Independent 8,478 6 G. Dean Hill - Independent 4,872 7 Steven J. Hooper - Independent 9,609 8 Aaron James - Independent 7,203 9 Elizabeth McLeod - Independent 16,652 10 Kacey Morgan - Independent 9,598 11 Eric William Stansberry - Independent 6,781 12 Write-In - John A. Gentry 64 13 Write-In - Al Green 0 Total Votes 2,959,761 December 2, 2020 State of Tennessee November 3, 2020 State General United States House of Representatives District 1 1 Diana Harshbarger - Republican 228,181 2 Blair Walsingham - Democratic 68,617 3 Steve Holder - Independent 8,621 4 Write-In - Josh Berger 4 Total Votes 305,423 United States House of Representatives District 2 1 Tim Burchett - Republican 238,907 2 Renee Hoyos - Democratic 109,684 3 Matthew L. -
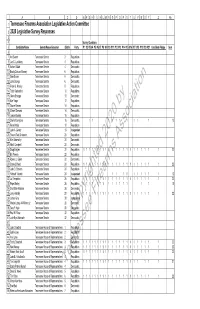
TFA-2020-Legislative-Survey-Results
ABCDEFGHIJKLMNOPQRSTUVWXYZAA 1 Tennessee Firearms Association Legislative Action Committee 2 2020 Legislative Survey Responses 3 4 Survey Questions 5 Candidate Name Senate/House/Governor District Party #1 #2 #3 #4 #5 #6 #7 #8 #9 #10 #11 #12 #13 #14 #15 #16 #17 #18 #19 #20 #21 Candidate Pledge Sum 6 7 Art Swann Tennessee Senate 2 Republican 8 Jon C. Lundberg Tennessee Senate 4 Republican 9 Amber Riddle Tennessee Senate 4 Democratic 10 Becky Duncan Massey Tennessee Senate 6 Republican 11 Sam Brown Tennessee Senate 6 Democratic 12 Jane George Tennessee Senate 6 Democratic 13 Frank S. Niceley Tennessee Senate 8 Republican 14 Todd Gardenhire Tennessee Senate 10 Republican 15 Glenn Scruggs Tennessee Senate 10 Democratic 16 Ken Yager Tennessee Senate 12 Republican 17 Shane Reeves Tennessee Senate 14 Republican by 18 Chase Clemons Tennessee Senate 14 Democratic 19 Janice Bowling Tennessee Senate 16 Republican 20 Sheila Younglove Tennessee Senate 16 Democratic 1 1 1111 1 11 1 10 21 Ferrell Haile Tennessee Senate 18 Republican 22 John A. Gentry Tennessee Senate 18 Independent 23 Steven Reid Dickerson Tennessee Senate 20 Republican 24 Kimi Abernathy Tennessee Senate 20 Democratic 2020 Association 25 Heidi Campbell Tennessee Senate 20 Democratic 26 Doug Englen Tennessee Senate 22 Republican 1 11111111 111111111111 1 22 27 Bill Powers Tennessee Senate 22 Republican 28 Ronnie L. Glynn Tennessee Senate 22 Democratic 29 Casey L Hood Tennessee Senate 24 Republican 1 11111111 111111111111 1 22 30 John D. Stevens Tennessee Senate 24 Republican 31 Yahweh Yahweh Tennessee Senate 24 Independent 1 1 1 1 1 1 11111 1 12 32 Jai Templeton Tennessee Senate 26 Republican 1 11111111 111111111111 1 22 33 Page Walley Tennessee Senate 26 Republican 1 1 1 1 1 1 1 111111 1 1 15 34 Civil Miller-Watkins Tennessee Senate 26 Democratic 35 Joey Hensley Tennessee Senate 28 Republican 1 11111111 111111111111 1 22 36 James Gray Tennessee Senate 28 Independent Firearms 37 Marion Latroy A-Williams Jr. -

Wedded to Wasting Time
VIEW FROM THE HILL Wedded to wasting time Is legislative action needed to protect clergy from same- sex nuptials? Experts say no. REALTY CHECK Sliding into P3 a new home If real estate deals had DAVIDSONLedger • WILLIAMSON • SUMNER • CHEATHAM • RUTHERFORD WILSON ROBERTSON • MAURY • DICKSON • MONTGOMERYumpires, • KNOX • ANDERSONthere might •BLOUNT be fewer•SEVIER brushbacks and balks. P3 25 years after hitting rock bottom, July 10 – 16, 2015 The power of information.NASHVILLE Vol. 41 EDITION | a new Nashville has emerged Issue 28 www.TNLedger.com Stories by | FORMERLY WESTVIEW SINCE 1978 Tim Ghianni begin on page 2 Page 13 Dec.: Nashville Public Library, Dec.: Keith Turner, Ratliff, Jeanan Mills Stuart, Resp.: Kimberly Dawn Wallace, Atty: Sheriff FateSpecial Thomas Collections Mary C Lagrone, 08/24/2010, 10P1318 In re: Jeanan Mills Stuart, Princess Angela Gates, Jeanan Mills Stuart, Princess Angela Gates,Dec.: Resp.: Kim Prince Patrick, Angelo Terry Patrick, pleaded guilty to mail Gates, Atty: Monica D Edwards, 08/25/2010, 10P1326 fraud, theft of In re: Keith Turner, TN Dept Of Correction, www.westviewonline.com TN Dept Of Correction, Resp.: Johnny Moore,Dec.: Melinda Atty: Bryce L Tomlinson, Coatney, Resp.: government property Pltf(s): Rodney A Hall, Pltf Atty(s): n/a, 08/27/2010, 10P1336 In re: Kim Patrick, Terry Patrick, Pltf(s): Sandra Heavilon, Resp.: Jewell Tinnon, Atty: Ronald Andre Stewart, 08/24/2010,Dec.: Seton Corp and tax conspiracy on 10P1322 Insurance Company, Dec.: Regions Bank, Resp.: Leigh A Collins, In re: Melinda L Tomlinson, -

2014 Report of Political Financial Support
2014 2014 Lilly Political Contributions As a biopharmaceutical company that treats serious diseases, Lilly plays an important role in public health and its related policy debates. It is important that our company shapes global public policy debates on issues specific to the people we serve and to our other key stakeholders including shareholders and employees. Our engagement in the political arena helps address the most pressing issues related to ensuring that patients have access to needed medications—leading to improved patient outcomes. Through public policy engagement, we provide a way for all of our locations globally to shape the public policy environment in a manner that supports access to innovative medicines. We engage on issues specific to local business environments (corporate tax, for example). Based on our company’s strategy and the most recent trends in the policy environment, our company has decided to focus on three key areas: innovation, health care delivery, and pricing and reimbursement. More detailed information on key issues can be found in our 2014 Corporate Responsibility Update. Through our policy research, development, and stakeholder dialogue activities, Lilly develops positions and advocates on these issues. Government actions such as price controls, pharmaceutical manufacturer rebates, and access to Lilly medicines affect our ability to invest in innovation. Lilly has a comprehen- sive government relations operation to have a voice in the public policymaking process at the federal, state, and local levels. Lilly is committed to participating in the political process as a responsible corporate citizen to help inform the U.S. debate over health care and pharmaceutical innovation.