Question Bankpo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Madhya Pradesh Lok Sabha General Elections 2009
Madhya Pradesh Lok Sabha General Elections 2009 Parliamentary Constituency 1 - MORENA Electors Polling Nominations Returning Officer's Dates Station Details Male 736596 55.04% 1529 Received 30 Name SHRI M.K. AGRAWAL 30-Apr-09 Female 601581 44.96% Rejected 1 Tel. Office 223500 Poll Total 1338177 16-May-09 Withdrawn 5 Tel. Res. 223400 Counting Contestant 24 Mobile No 9826263270 Result 16-May-09 S.No. Name of the Candidate Party Votes Secured Result Number Per 1 Narendra Singh Tomar Bharatiya Janata Party 300647 42.30 Winner 2 Ramniwas Rawat Indian National Congress 199650 28.09 Nearest 3 Balveer Singh Dandotiya Bahujan Samaj Party 142073 19.99 Lost 4 Ad. Baijnath Kushwaha Samajwadi Party 32129 4.52 DF 5 Jugal Kishor Pippal Communist Party of India (Marxist) 5537 0.78 DF 6 Rajveer Singh Independent 3438 0.48 DF 7 Kalwati Ramesh Argal Independent 2977 0.42 DF 8 Narendra Singh Independent 2926 0.41 DF 9 Anita Hitendra Choudhary Bhartiya Bahujan Samaj Party 2377 0.33 DF 10 Mahesh Singh Jatav Independent 2052 0.29 DF 11 Dhallu Allahbaksh Independent 1844 0.26 DF 12 Vivek Apte Independent 1534 0.22 DF 13 Devendra Singh Sikarwar All India Forward Bloc 1520 0.21 DF 14 Ram Sewak Independent 1455 0.20 DF 15 Jogendr Independent 1425 0.20 DF 16 Satyendra Jain Shammi Independent 1410 0.20 DF 17 Mahesh Jatav Independent 1323 0.19 DF 18 Ramniwas Kushwah Independent 1101 0.15 DF 19 Gandrv Independent 958 0.13 DF 20 Vijay Kumar Independent 916 0.13 DF 21 Uttam Singh Mittal Independent 877 0.12 DF 22 Usha Rawat Independent 873 0.12 DF 23 Vishanlal Agrawal Gokal M.P. -

LOK SABHA DEBATES (English Version)
Tenth Series, Vol. XLV, No.1 Monday, November 27, 1995 Agrahayana 6,1917 (Saka) LOK SABHA DEBATES (English Version) Fifteenth Session (Tenth Lok Sabha) (Vol. XLV contains Nos. J to 10) LOK SABRA SECRETARIAT NEW DELHI Price: Rs. 5Q.OO CONTENTS [Tenth Series, Vol. XLV, Fifteenth Session, 199511917 (Saka)] No.1, Monday, November 27, 1995/Agrahayana 6, 1917 (Saka) COlUMNS ALPHABETICAL LIST OF MEMBERS i-xi OFFICERS OF THE LOK SABHA xiii COUNCIL OF MINISTERS xv-xviii NATIONAL. ANTHEM - Played !NTRODUCTION OF MINISTERS 1-4 OBITUARY REFERENCES 5-17 WRITTEN ANSWERS TO QUESTIONS 18-378 Starred Questions 1-20 18-55 Unstarred Questions 1-182 55-378 ALPHABETICAL LIST OF MEMBERS TENTH LOK SABHA A B Abdul Ghafoor, Shri (Gopalganj) Baitha, Shri Mahendra (Bagaha) Abedya Nath, Mahant (Gorakhpur) Bala Dr, Asim (Nabadwip) Acharia, Shri 8asudeb (Bankura) Balayogi, Shri G.M.C. (Amalapuram) Adaikalaraj, Shri L. (Tiruchirapalli) 8aliyan, Shri N.K. (Muzaffamagar) Bandaru, Shri Dattatraya (Secunderabad) Advani, Shri Lal K. (Gandhi Nagar) Banerjee. Kumari Mamata (Calcutta South) Agnihotri, Shri Rajendra (Jhansi) Bansal. Shri Pawan Kumar (Chandigarh) Ahmed. Shri E. (Manjeri) Barman, Shri Palas (Balurghat) Ahirwar. Shri Anand (Sagar) Barman, Shri Uddhab (Barpeta) Ahmed, Shri Kamaluddin (Hanamkonda) Basu, Shri Anil (Arambagh) Aiyar. Shri Mani Shankar (Mayiladuturai) Basu, Shri Chitta (Barasat) Ajit Singh, Shri (Baghpat) Berwa. Shri Ram Narain (Tonk) Akber Pasha, Shri B. (Vellore) Bhadana, Shri Avtar Singh (Faridabad) Amar Pal Singh, Shri (Meerut) Bhagat, Shri Vishweshwar (Balaghat) Anbarasu, Shri R. (Madras Central) Bhakta, Shri Manoranjan (Andaman and Nicobar Anjalose, Shri Thayil John (Alleppey) Island) Ansari, Dr. Mumtaz (Kodarma) Bhandari, Shrimati Oil Kumari (Sikkim) Antulay. -

Alphabetical List of Recommendations Received for Padma Awards - 2014
Alphabetical List of recommendations received for Padma Awards - 2014 Sl. No. Name Recommending Authority 1. Shri Manoj Tibrewal Aakash Shri Sriprakash Jaiswal, Minister of Coal, Govt. of India. 2. Dr. (Smt.) Durga Pathak Aarti 1.Dr. Raman Singh, Chief Minister, Govt. of Chhattisgarh. 2.Shri Madhusudan Yadav, MP, Lok Sabha. 3.Shri Motilal Vora, MP, Rajya Sabha. 4.Shri Nand Kumar Saay, MP, Rajya Sabha. 5.Shri Nirmal Kumar Richhariya, Raipur, Chhattisgarh. 6.Shri N.K. Richarya, Chhattisgarh. 3. Dr. Naheed Abidi Dr. Karan Singh, MP, Rajya Sabha & Padma Vibhushan awardee. 4. Dr. Thomas Abraham Shri Inder Singh, Chairman, Global Organization of People Indian Origin, USA. 5. Dr. Yash Pal Abrol Prof. M.S. Swaminathan, Padma Vibhushan awardee. 6. Shri S.K. Acharigi Self 7. Dr. Subrat Kumar Acharya Padma Award Committee. 8. Shri Achintya Kumar Acharya Self 9. Dr. Hariram Acharya Government of Rajasthan. 10. Guru Shashadhar Acharya Ministry of Culture, Govt. of India. 11. Shri Somnath Adhikary Self 12. Dr. Sunkara Venkata Adinarayana Rao Shri Ganta Srinivasa Rao, Minister for Infrastructure & Investments, Ports, Airporst & Natural Gas, Govt. of Andhra Pradesh. 13. Prof. S.H. Advani Dr. S.K. Rana, Consultant Cardiologist & Physician, Kolkata. 14. Shri Vikas Agarwal Self 15. Prof. Amar Agarwal Shri M. Anandan, MP, Lok Sabha. 16. Shri Apoorv Agarwal 1.Shri Praveen Singh Aron, MP, Lok Sabha. 2.Dr. Arun Kumar Saxena, MLA, Uttar Pradesh. 17. Shri Uttam Prakash Agarwal Dr. Deepak K. Tempe, Dean, Maulana Azad Medical College. 18. Dr. Shekhar Agarwal 1.Dr. Ashok Kumar Walia, Minister of Health & Family Welfare, Higher Education & TTE, Skill Mission/Labour, Irrigation & Floods Control, Govt. -

“All the Advocates/Litigants Are Informed That in View of the Directions Dated 09.03.2021 Passed by Hon. DB-II in W.P.(C) 2018
19.03.2021 “All the Advocates/Litigants are informed that in view of the directions dated 09.03.2021 passed by Hon. DB-II in W.P.(C) 2018/2021 and W.P.(C) 2673/2021 half of the cases (starting from the Supplementary List/s) listed for a particular day shall be taken up in the Pre-lunch Session and rest of the cases shall be taken up in the Post- lunch Session. All the Advocates/Litigants may accordingly reach the Court Rooms according to the turn of their case/s in order to curtail the number of people in court premises at the same time.” NOTE 1. CAUSE LIST OF CASES FILED BETWEEN 01.01.2018 TO 21.03.2020 SHALL NOT BE PUBLISHED TILL FURTHER ORDERS. DELETION NOTE 1. W.P.(CRL.) 571/2021 LISTED BEFORE HON'BLE DB-III AT ITEM NO.1 IS DELETED AS THE SAME IS LISTED BEFORE HON'BLE DB-I. 2. W.P.(C) 3408/2019 LISTED BEFORE HON'BLE DB-V AT ITEM NO.4 IS DELETED AS THE SAME IS LISTED BEFORE HON'BLE DB-IV. 3. BAIL APPL. 672/2021 LISTED BEFORE HON'BLE MS. JUSTICE MUKTA GUPTA AT ITEM NO.18 IS DELETED AS THE SAME IS LISTED BEFORE HON'BLE MS. JUSTICE ANU MALHOTRA. 4. FAO 206/2004 LISTED BEFORE HON'BLE MR. JUSTICE NAJMI WAZIRI AT ITEM NO.8 IS DELETED 5. W.P.(C) 1036/2020 LISTED BEFORE HON'BLE MR. JUSTICE V. KAMESWAR RAO AT ITEM NO.2 IS DELETED AS THE SAME IS FIXED FOR 19.04.2021. -

Hockey Year Book
K. Arumugam’s International HOCKEY YEAR BOOK NEWSLETTER October 2007 Issue 2 Mark my Words Things are looking bright for Indian hockey. Yes, we are sad that we missed the Beijing Olympics, but we know single failure is not final. Our hockey can bounce back on the strength of national passion. We have to think afresh, re-orient priorities, policies and perception on processes and personalities so that disasters of such nature doesn’t recur. Thankfully, the Indian Olympic Association is Field Hockey Publications brought out Balbir Singh Sr.’s Golden Yardstick. This book seized of the matters. With the IOA at the was released by Hon’ble Prime Minister of India, Manmohan Singh on 2nd June 2008. helm, it is expected things will move on purposefully. At Field Hockey Publications, we are coming out with our Hockey Year Book 2008 shortly. It will be totally a domestic affair. We have also completely re-designed our popular website, stick2hockey.com, with a slew of new features such as player profiles, slide shows, newspaper search and many more. K. Arumugam Author www.stick 2hockey.com Latest News visit Women Hockey stick School Hockey 2 Chak De News hockey.com HJS Chimni Column Expert Eye news updates Domestic Hockey player profile Latest News Player Corner Women Hockey FIH News slide shows School Hockey Interviews PicWorld Affairs Chak De News PHL News Live Commentary HJS Chimni Column Media Watch Expert Eye IHF Watch plenty more Domestic Hockey History Watch Player Corner Book Watch FIH News Statistics Interviews Current Players Profiles PHL -

6President of India Gold
follow us: monday, september 9, 2019 Delhi City Edition thehindu.com 24 pages ț ₹10.00 facebook.com/thehindu twitter.com/the_hindu Printed at . Chennai . Coimbatore . Bengaluru . Hyderabad . Madurai . Noida . Visakhapatnam . Thiruvananthapuram . Kochi . Vijayawada . Mangaluru . Tiruchirapalli . Kolkata . Hubballi . Mohali . Malappuram . Mumbai . Tirupati . lucknow . cuttack . patna NEARBY Trump calls off secret talks with Taliban Chandrayaan2 orbiter U.S. President killed a U.S. soldier and 11 others in Kabul. “I imme criticises bomb diately cancelled the meet attack in Kabul ing and called off peace ne spots lander on moon gotiations,” he wrote. Michael Crowley “If they cannot agree to a ISRO chief Sivan confirms hard landing of module Lara Jakes ceasefire during these very Mujib Mashal important peace talks, and Press Trust of India Mob attacks policemen Washington in Muzaffarpur district would even kill 12 innocent BENGALURU PATNA President Donald Trump people, then they probably Chandrayaan2’s lander Vik A video in which a group of said on Saturday that he had don’t have the power to ne ram has been located on the villagers are seen thrashing cancelled a secret meeting at gotiate a meaningful agree lunar surface and it must some policemen in Camp David with Taliban ment anyway,” Mr. Trump have been a hardlanding, Muzaffarpur district of Bihar leaders and the President of added. “How many more de ISRO Chairman K. Sivan said went viral on social media on Afghanistan and was calling A trigger: A U.S. soldier was one of the victims of the cades are they willing to on Sunday. -
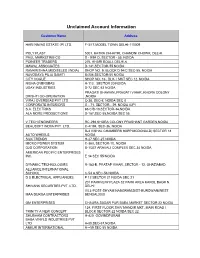
Unclaimed Account Information
Unclaimed Account Information Customer Name Address HARI NIWAS ESTATE (P) LTD. F-3/17,MODEL TOWN DELHI-110009 POLY PLAST 5001, KATRA CHHATRI, CHANDNI CHOWK, DELHI. PAUL MARKETING CO S - 9/94 D, SECTOR - 55, NOIDA POINEER TRADERS 276, KHARI BOALI, DELHI-6 MAWAL ASSOCIATES D-141,SECTOR-55 NOIDA RAMKRISHNA MEDISELES (INDIA) SHOP NO. 9, BLOCK D 94/C SEC-55, NOIDA NAVODAYA PUJA SAMITI B-236 SECTOR-55 NOIDA CITY VOGUE SHOP NO. 16 , BLK- I MKT SEC 12, NOIDA NISHA OVERSEAS A-113 , SECTOR 20 NOIDA UDAY INDUSTRIES D-72 SEC 63 NOIDA PRAGATI BHAWAN ,PRAGATI VIHAR ,KHORA COLONY DRISHTI CO-OPERATION ,NOIDA VIRAJ OVERSEAS PVT LTD C-38, SEC-8, NOIDA SEC 8 CORPORATE INTERIORS G - 71, SECTOR - 39, NOIDA (UP) S.A. ELECTRIKS 86 C/B-10,SECTOR-34,NOIDA ALA MORE PRODUCTIONS D-187,SEC-55,NOIDA SEC 55 V.TECH.ENGINEERS RC-298,KHORA COLONY PRASHANT GARDEN,NOIDA BEAUSOFT INDIIA PVT. LTD. C-4/194, SEC-36, NOIDA B-4 VISHAL CHAMBERS 9OPP MCDONALD) SECTOR 18 AUTO WHEELS NOIDA SILK TRENDS H-27,SEC-27,NOIDA MICRO POWER SYSTEM C-383, SECTOR-10, NOIDA D2D CORPORATION B-1/337 ARAVALI COMPLEX SEC 34 NOIDA AMERICAN PECIFIC ENTERPRISES INC. E 14 SEC 55 NOIDA DYNAMIC TECHNOLOGIES R-183-B, PRATAP VIHAR, SECTOR - 12, GHAZIABAD ALLIANCE INTERNATIONAL SCHOOL C-54 A,SEC-56,NOIDA D S ELECTRICAL APPLIANCES F 12 SECTOR 21 NOIDA SEC 21 201 PARKVEIW PLAZA 32 PARK AREA KAROL BAGH N. SHIVANK SECURITIES PVT. LTD. DELHI VILL-POST-SHYAM NABGRAM,DIST-BURDWAN(WEST MAA DURGA ENTERPRISES BENGAL)000 OM ENTERPRISES CHAURA SADAR PUR BABA MARKET SECTOR 22 NOIDA 124, FIRST FLOOR SHIV MANDIR MKT. -

SIKH TIMES WEBSITE PAGE.Qxd
instagram.com/ @thesikhtimes facebook.com/ thesikhtimes qaumipatrika VISIT: PUBLISHED FROM Delhi, Haryana, Uttar www.thesikhtimes.in Pradesh, Punjab, The Sikh Times Email:[email protected] Chandigarh, Himachal and Jammu National Daily Vol. 12 No. 223 RNI NO. DELENG/2008/25465 New Delhi, Saturday, 2 January, 2021 [email protected] 9971359517 12 pages. 2/- Simmi Kaur Babbar ÁŒÀ‹Ë ∑‘§ ’ÊÚ«¸⁄UÙ¥ ¬⁄U ’ÒΔ ‹ÊπÙ¥ Á∑§‚ÊŸÙ¥ ∑‘§ ‚ÊÕ ∑§Ù߸ ŸÃÊ ÿÊ ‚⁄U∑§Ê⁄U œÙπÊ Ÿ„Ë¥ Oxford-AstraZeneca Covid vaccine set to become first to get approval in India New Delhi, January 1: An expert panel on COVID-19 of the Central Drugs Standard Control Organisation (CDSCO) is set to recom- mend granting emergency use authorisation for the Oxford COVID-19 vaccine Covishield, being manufactured by Serum Institute of India, sources said on Friday.The Pune-based Serum ∑§⁄U ‚∑§ÃË– Institute of India (SII), the world’s largest vac- cine manufacturer, has tied up with AstraZeneca to manufacture Covishield. The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) on Wednesday had approved the COVID-19 vaccine developed by scientists at Oxford University and produced by AstraZeneca for human use.The Subject Á∑§‚ÊŸÙ¥ ‚ ªgÊ⁄UË ∑§⁄UŸ flÊ‹Ê Expert Committee (SEC) on COVID-19 of the CDSCO, which had earlier sought additional safety and immunogenicity data from SII, delib- erated on its application seeking emergency use authorisation (EUA) for the shots on Wednesday, and met again on Friday to review ’ŇÊÊ Ÿ„Ë¥ ¡Ê∞ªÊ– the matter. After SII’s application, the SEC has started reviewing the EUA application by Bharat Biotech for its COVID-19 vaccine Covaxin but is yet to take a final decision on the Gurcharan Singh Babbar matter, sources said. -

Olympian Aslam Sher Khan Offers to Be Super-Sub for Rahul Gandhi Not
hange P C ro -X d F u D c t P Imphal Times Supplementary issue Page 3 w w m w Click to buy NOW! o . .c tr e ac ar ker-softw‘JD(S) leaders should be ready’: Nikhil New Hope for Breast Cancer Patients shown promise for treatment Immunomodulators Kumaraswamy on assembly polls of advanced and metastatic manipulate the “brakes” and breast cancer. “gas pedals” of the immune Age ncy fiercely fought contest, Targeted Therapy: system. The immune system Bengaluru June 7, however, said there was no Targeted therapies target the is guarded by checkpoints threat to the government and his cancer’s specific genes, to self-regulate itself. Indicating ‘lack of trust’ father will complete the tenure. proteins, or the tissue Checkpoint inhibitors between the coalition partners, “There is no problem to the environment that contributes modify the immune Karnataka Chief Minister H D government. It will complete (its to cancer growth and survival. responses against cancer Kumaraswamy’s son Nikhil tenure). You get tensed due to When it comes to breast cells. Kumaraswamy, in a viral video, reports in media. It is not like that. cancers some subtypes have Oncolytic virus therapy: is purportedly seen asking We know what is there inside too much of a protein called Viruses like adenovirus, JD(S) workers to prepare (the government). Kumaranna human epidermal growth herpes simplex virus, themselves for the assembly (Kumaraswamy) will run (the Dr Meenu Walia, Director- factor receptor 2 (HER2, Maraba virus, etc are known polls. government) for the next four Medical Oncology, Max pronounced her-too). -

Dow Chemical/Olympics: Simon Hughes Signs up to Campaign As Jowell Demands Answers
Trial by Jeory Page 1 of 5 Dow Chemical/Olympics: Simon Hughes signs up to campaign as Jowell demands answers November 22, 2011 by trialbyjeory (photo copyright: Ted Jeory) Dow Chemical’s sponsorship of the Olympic stadium, which I’ve been writing about pretty much alone in the UK press since the deal with Locog was done in August, is starting to enter serious political territory. Here’s a summary of three latest developments on the controversial wrap, (pictured above, temporarily attached to the stadium last week): 1. Lib Dem deputy leader Simon Hughes has today signed up to the cross-[arty campaign to force Locog to reconsider its decision. he has added his name to a letter that has been sent to Lord Coe, which I’ll detail below. Tory MP Priti Patel is also on board. 2. Shadow Olympics Minister (& Olympics Board member) Tessa Jowell has stepped up her own rhetoric, a) by writing to the Chair of the Commission for a Sustainable London 2012 to demand disclosure of important tender documents around the deal; and b) asking for a meeting with Dow themselves. 3. Locally, Olympic host borough Tower Hamlets Council is expected to lodge a formal objection with Locog about the deal after a vote next week. Here is some more detail about the developments today: 1. Simon Hughes has added his name to the following letter to Lord Coe: Dear Lord Coe, http://trialbyjeory.wordpress.com/ 11/23/2011 Trial by Jeory Page 2 of 5 We the undersigned call on the London Organising Committee of the Olympic Games to review its decision in August 2011 to award the Olympic wrap tender to Dow Chemical Company in light of its appalling human rights record in regard to the victims of the 1984 Bhopal disaster. -

Lok Sabha Debates
LOK SABHA DEBATES Vol. I First day of the First Session of the Tenth Lok Sabha No. 1. LOK SABHA 11.01 hrs. WELCOME OF THE MEMBERS OF Tuesday. July 9, m i/A sa d h18, a 1913 NEW LOK SABHA BY SPEAKER (Saka) Pro tem Laid on the Table The Lok Sabha met at Eleven of the Clock[English] MR. SPEAKER: It gives me great pleasure to welcome all the Members who [The Speaker pro tem (SHRI INDRAJIT GUPTA) in the Chair]. have been elected to the Tenth Lok Sabha. I am sure you will all help the Chair in maintaining the high traditions of this OBSERVANCE OF SILENCE House and thereby strengthening the roots [Englirii] of parliamentary democracy in our country. MR. SPEAKER: We are meeting to I wish you all success in your endea day on a solemn occasion. A new Lok vours. Sabha has been elected under the Con stitution charged with great and heavy 11.02 hrs. responsibilities for the welfare erf the LIST OF MEMBERS ELECTED TO LOK country and our people. SABHA It is fit and proper, as is customary on L ad on the Table such an occasion, that we all stand silence [English] for a short while before we begin our proceedings. SECRETARY GENERAL : Sir, I lay on the Table a Book presented to the The Members then stood hi silence for Speaker by the Chief Election Commis a short while. sioner, containing the list of Members elected to the Lok Sabha at the general elections of 1991 : LIST OF MEMBERS ELECTED TO LOK SABHA No. -
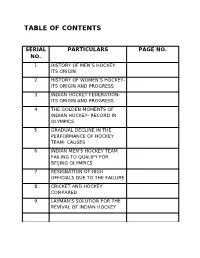
Table of Contents
TABLE OF CONTENTS SERIAL PARTICULARS PAGE NO. NO. 1 HISTORY OF MEN’S HOCKEY- ITS ORIGIN 2 HISTORY OF WOMEN’S HOCKEY- ITS ORIGIN AND PROGRESS 3 INDIAN HOCKEY FEDERATION- ITS ORIGIN AND PROGRESS 4 THE GOLDEN MOMENTS OF INDIAN HOCKEY- RECORD IN OLYMPICS 5 GRADUAL DECLINE IN THE PERFORMANCE OF HOCKEY TEAM- CAUSES 6 INDIAN MEN’S HOCKEY TEAM FAILING TO QUALIFY FOR BEIJING OLYMPICS 7 RESIGNATION OF HIGH OFFICIALS DUE TO THE FAILURE 8 CRICKET AND HOCKEY COMPARED 9 LAYMAN’S SOLUTION FOR THE REVIVAL OF INDIAN HOCKEY OBJECTIVE Every work is and should be supported by an objective. Without a specific objective that particular work is of no use. Therefore every project work should have something to say or teach people who are reading it. Here the topic of discussion is the “JOURNEY OF INDIAN HOCKEY”. We are here to discuss about how hockey evolved as a world famous game, how it came to India and the way the Indians mastered it. But we have to consider the slowdown in the performance of Indian hockey teams in the last 2-3 decades. It has really shown a downward trend. Then we will look upon the causes for the decline and the measures for revival of Indian hockey. My sole objective to make a project report on this topic is to create awareness among my mates and the people, about HOCKEY the sport which the people of our country have forgotten. People don’t even know the names of hockey players and the tournaments in which the team plays at various levels.