Desloratadine (Clarinex)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

22-556Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 22-556Orig1s000 MEDICAL REVIEW(S) MEDICAL OFFICER REVIEW Division Of Pulmonary, Allergy, and Rheumatology Products, HFD-570 APPLICATION: NDA 22-556 TRADE NAME: Karbinal ER™ APPLICANT/SPONSOR: Tris Pharma USAN NAME: Carbinoxamine Extended-Release MEDICAL OFFICER: Peter Starke, MD Oral Suspension TEAM LEADER: Theresa Michele, MD CATEGORY: Antihistamine DATE: February 25, 2013 ROUTE: Oral SUBMISSIONS REVIEWED IN THIS DOCUMENT Document Date Submission Date Application/Doc Comments October 4, 2012 October 5, 2012 SD-17 Complete Response submission January 8, 2013 January 9, 2013 SD-20 Response to labeling (formatting) IR RELATED APPLICATIONS Date Application Comments REVIEW SUMMARY: This is clinical review of a Complete Response (CR) to a CR action taken by the Agency on October 7, 2011, for a 505(b)(2) application from Tris Pharma for Carbinoxamine Extended-Release (ER) Oral Suspension, equivalent to 4 mg of carbinoxamine maleate (CM) per 5 mL. The formulation is a sustained release formulation of carbinoxamine maleate suspended in a drug-polistirex resin complex. The proposed Trade Name is Karbinal ER. The application references both the currently available generic immediate-release Carbinoxamine Maleate 4 mg tablets (ANDA 40-442) and oral solution 4 mg/5 mL(ANDA 40-458), marketed under the brand name Palgic and manufactured by Milkart, Inc., and the no-longer-marketed immediate-release innovator products, Clistin 4 mg tablets (NDA 08-915) and 4 mg/5 mL elixir (NDA 08-955), previously marketed by McNeil. McNeil discontinued marketing the Clistin products in the 1990s, and the Orange Book makes the notation that the Clistin products were not discontinued or withdrawn for safety or efficacy reasons. -

FEXOFENADINE HYDROCHLORIDE 180 MG FILM-COATED TABLETS Fexofenadine Hydrochloride
ID1089 MRP _ UK Version: 07 Review Date: 19/02/2020 PACKAGE LEAFLET: INFORMATION FOR THE USER FEXOFENADINE HYDROCHLORIDE 120 MG FILM-COATED TABLETS FEXOFENADINE HYDROCHLORIDE 180 MG FILM-COATED TABLETS Fexofenadine hydrochloride Read all of this leaflet carefully before you start using this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any of the side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet, (see section 4). In this leaflet: 1. What Fexofenadine hydrochloride is and what it is used for 2. What you need to know before you take Fexofenadine hydrochloride 3. How to take Fexofenadine hydrochloride 4. Possible side effects of Fexofenadine hydrochloride 5. How to store Fexofenadine hydrochloride 6. Contents of the pack and other information 1. WHAT FEXOFENADINE HYDROCHLORIDE IS AND WHAT IT IS USED FOR FEXOFENADINE HYDROCHLORIDE Contains fexofenadine hydrochloride which is an antihistamine. Only Fexofenadine hydrochloride 120 mg tablets is used in adults and adolescents of 12 years and older to relieve the symptoms that occur with hay fever (seasonal allergic rhinitis) such as sneezing, itchy, running or blocked nose and itchy, red and watery eye). Fexofenadine hydrochloride 180 mg tablets is used in adults and adolescents of 12 years and older to relieve the symptoms that occur with long term allergic skin reactions( chronic idiopathic urticaria) such as itching, swelling and rashes 2. -

Download a Drug Interactions Card
transplant.bc.ca/medications Please discuss with your healthcare professionals BEFORE starting or stopping any medications, herbal or non-prescription products. Contact your Transplant Clinic nurse or pharmacist to let them know if there are any changes to your medications. Transplant Clinic Phone: _____________________________________ BC PHN (CareCard #) __________________________________ (2017v3) Please call your transplant clinic before starting any new medications to avoid possible drug interactions, especially those with CAUTION (see below) next to its name: Cyclosporine (Neoral), Tacrolimus (Prograf/Sandoz tac, Advagraf), Sirolimus (Rapamune): Seizure: phenytoin, carbamazepine, phenobarbital, primidone Infection: erythromycin, clarithromycin – CAUTION ( OK- azithromycin) fluconazole, ketoconzazole, posaconazole voriconazole - CAUTION rifampin – CAUTION Cyclosporine (Neoral), Tacrolimus (Prograf/Sandoz tac, d Advagraf), Sirolimus (Rapamune) cont’d Depression: fluoxetine, fluvoxamine ( OK- paroxetine, citalopram, escitalopram, sertraline, venlafaxine, mirtazapine) Heart/Blood pressure: diltiazem, verapamil, amiodarone, digoxin Cholesterol: lovastatin, simvastatin, atorvastatin ( OK- rosuvastatin, pravastatin, fluvastatin) Pain: anti-inflammatories can affect kidney function: ibuprofen, naproxen, diclofenac, indomethacin, celecoxib ( OK- acetaminophen) Mycophenolate mofetil/sodium (MMF, Cellcept/Myfortic): Antacids: space taking antacid and MMF by 2 hours Cholestyramine: AVOID if possible Azathioprine (Imuran): Gout: allopurinol – -

PACKAGE LEAFLET: INFORMATION for the PATIENT Desloratadine 5
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Desloratadine 5 mg tablets Read all of this leaflet carefully before you start taking/using this medicine because it contains important information for you. Keep this leaflet. You may need to read it again. If you have any further questions, ask your doctor, pharmacist or nurse. This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What Desloratadine 5 mg Tablets are and what they are used for 2. What you need to know before you take Desloratadine 5 mg Tablets 3. How to take Desloratadine 5 mg Tablets 4. Possible side effects 5. How to store Desloratadine 5 mg Tablets 6. Contents of the pack and other information 1. What Desloratadine 5 mg Tablets are and what they are used for What Desloratadine 5 mg Tablets is Desloratadine 5 mg Tablets contain desloratadine which is an antihistamine. How Desloratadine 5 mg Tablets works Desloratadine 5 mg Tablets is an antiallergy medicine that does not make you drowsy. It helps control your allergic reaction and its symptoms. When Desloratadine 5 mg Tablets should be used Desloratadine relieves symptoms associated with allergic rhinitis (inflammation of the nasal passages caused by an allergy, hay fever or allergy to dust mites) in adults and adolescents 12 years of age and older. -
Fexofenadine Hydrochloride) Tablets
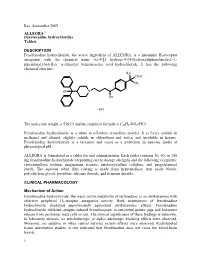
Rev. September 2005 ALLEGRA® (fexofenadine hydrochloride) Tablets DESCRIPTION Fexofenadine hydrochloride, the active ingredient of ALLEGRA, is a histamine H1-receptor antagonist with the chemical name (±)-4-[1 hydroxy-4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-butyl]-α, α-dimethyl benzeneacetic acid hydrochloride. It has the following chemical structure: The molecular weight is 538.13 and the empirical formula is C32H39NO4•HCl. Fexofenadine hydrochloride is a white to off-white crystalline powder. It is freely soluble in methanol and ethanol, slightly soluble in chloroform and water, and insoluble in hexane. Fexofenadine hydrochloride is a racemate and exists as a zwitterion in aqueous media at physiological pH. ALLEGRA is formulated as a tablet for oral administration. Each tablet contains 30, 60, or 180 mg fexofenadine hydrochloride (depending on the dosage strength) and the following excipients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and pregelatinized starch. The aqueous tablet film coating is made from hypromellose, iron oxide blends, polyethylene glycol, povidone, silicone dioxide, and titanium dioxide. CLINICAL PHARMACOLOGY Mechanism of Action Fexofenadine hydrochloride, the major active metabolite of terfenadine, is an antihistamine with selective peripheral H1-receptor antagonist activity. Both enantiomers of fexofenadine hydrochloride displayed approximately equipotent antihistaminic effects. Fexofenadine hydrochloride inhibited antigen-induced bronchospasm in sensitized guinea pigs and histamine -

Prescribing Information for High-Dose Fexofenadine in the Management of Urticaria in Adults
1 Prescribing information for high-dose fexofenadine in the management of urticaria in adults This prescribing information document outlines the prescribing responsibilities between the specialist and GP. GPs are invited to participate. If the GP feels that such prescribing is outside their area of expertise or have clinical concerns about the safe management of the drug in primary care, then he or she is under no obligation to do so. In such an event, clinical responsibility for the patient’s health remains with the specialist. If a specialist asks the GP to prescribe, the GP should reply to this request as soon as practicable. Consultant details GP details Patient details Name: Name: Name: Address: Address: NHS Number: Email: Email: Date of birth: Contact number: Contact number: Contact: Introduction Fexofenadine is a second generation non-sedating H1-antihistamine used in the treatment of allergic disorders. Fexofenadine is a pharmacologically active metabolite of terfenadine. Licensed indication: In adults and children 12 years and older, the licensed dose is 180mg daily for the relief of symptoms associated with chronic idiopathic urticaria. Unlicensed indication (the focus of this document): As advised by Europeani and British Association of Dermatologistsii guidelines, doses of antihistamines up to four times the recommended daily dose may be used in the treatment of urticaria. Adult dosage and administration The licensed maximum recommended daily dose is 180mg once daily taken before a meal. Doses of up to four times the daily recommended dose have been used in the treatment of severe urticaria (unlicensed indication)i Available as: 30mg, 120mg and 180mg tablets Specialist responsibilities Provide patient/carer with relevant written information on the unlicensed use of high-dose fexofenadine and possible side-effects. -

Alternative Forms of Oral Drug Delivery for Pediatric Patients Marcia L
PEDIATRIC PHARMACOTHERAPY A Monthly Newsletter for Health Care Professionals from the University of Virginia Children’s Hospital Volume 19 Number 3 March 2013 Alternative Forms of Oral Drug Delivery for Pediatric Patients Marcia L. Buck, Pharm.D., FCCP, FPPAG he lack of an appropriate dosage form the concentration versus time curve (AUC) of T limits the use of many medications that 45% for lopinavir and 47% for ritonavir (p = may potentially benefit children. While this has 0.003 and 0.006, respectively). been a long-standing problem for pediatric healthcare providers, little attention has been Cutting tablets, another common practice, may paid to remedying it until recently. In 2005 the be acceptable for some drugs, however this Eunice Kennedy Shriver National Institute for practice can introduce considerable variability Child Health and Human Development, joined between doses. In drugs with a narrow by representatives from the Food and Drug therapeutic index, such as levothyroxine, this Administration (FDA), academic medicine, and variability may be enough to produce clinically the pharmaceutical industry, formed the United significant changes in clinical response.7 When States (US) Pediatric Formulations Initiative in cutting a tablet is necessary, family members an effort to stimulate research in pediatric should receive specific instructions on the formulation technology.1 Similar work by the process, including the proper use of a tablet European Medication Agency (EMA) led to the splitter. Family members involved in dose development of the European Pediatric preparation should also understand how to Formulation Initiative.2 In addition, the World dispose of unused drug and the need to avoid Health Organization launched a global initiative repeated exposure to drugs that have in 2007 entitled “Make Medicines Child Size” to carcinogenic or teratogenic properties. -

Evaluation of the Efficacy of the Utilization of the Imipramine For
vv Clinical Group Archives of Otolaryngology and Rhinology ISSN: 2455-1759 DOI CC By Diaa El Din El Hennawi, Mohamed Rifaat Ahmed*, Yasser T Madian, Research Article Mohammed T El-Tabbakh and Ibrahim Hassan Ibrahim Evaluation of the efficacy of the Department of Otorhinolaryngology, Faculty of utilization of the imipramine for medicine Suez Canal University, Ismailia – Egypt Dates: Received: 13 May, 2017; Accepted: 22 July, patients with allergic rhinitis 2017; Published: 26 July, 2017 *Corresponding author: Mohamed Rifaat Ahmed, MD, Assistant Professor, Otolaryngology, Fac- ulty of Medicine, Suez Canal University, Ismailia, Abstract Egypt, Tel: +201285043825; Fax: +20663415603; E-mail: Background: allergic rhinitis is an infl ammatory process of the upper respiratory tract with a continuous symptom that last for hour or more with symptom affect patient’s work and quality of life. Keywords: Allergic rhinitis; Imipramine; Desloratadine; Randomized controlled trial Methodology: a randomized controlled trial in which we enrolled 284 patients with allergic rhinitis for periods longer than 4 days/week and for more than 4 consecutive weeks divided into two groups. https://www.peertechz.com Group A (n=142) received Imipramine 75 mg/day for 90 days .Group B (n=142), the control group, received desloratadine 5 mg / day for 90 days. Results: The mean visual analogue score nasal symptoms intensity (sneezing, Nasal obstruction, watery rhinorrhea and itchy nose) showed a statistically signifi cantly improvement in group A (treated with Imipramine) compared with group B (treated with desloratadine) after the same treatment period. Conclusion: Imipramine could be used in treatment patients with allergic rhinitis as it is effective as an antihistaminic besides its main action in reliving psychosocial stresses. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -

Medications That May Interfere with Skin Testing
MEDICATIONS THAT MAY INTERFERE WITH SKIN TESTING • Due to continued advances, not all medications may be listed at time of printing. • For your safety and accurate results, at each visit, please list all your current medications (including non-prescription and those prescribed elsewhere). • It is important to let us know if you are pregnant or could be pregnant. STOP THESE MEDICATIONS FIVE DAYS BEFORE SKIN TESTING: ORAL ANTIHISTAMINES: ANTIHISTAMINE NOSE SPRAYS: • Allegra (Fexofenadine) • Astelin, Astepro, Dymista (Azelastine) • Benadryl (Diphenhydramine) • Patanase (Olopatadine) • Claritin, Alavert (Loratadine) • Clarinex (Desloratadine) ANTIHISTAMINE EYE DROPS: • Xyzal (Levocetirizine) • Alaway, Claritin, Zaditor, Zyrtec (Ketotifen) • Zyrtec (Cetirizine) • Bepreve (Bepotastine) • Cyproheptadine • Elestat (Epinastine) · All over-the-counter medications for allergy, cough, cold, sleep, • Emadine (Emedastine) or nausea that include: • Lastacaft (Alcaftadine) oAcrivastine (ex. Semprex) • Livostin (Levocabastine) oAzatadine (ex. Optimine, Trinalin) • Naphcon-A, Opcon-A, Visine-A oBrompheniramine (ex. Dimetapp) (Pheniramine) oCarbinoxamine (ex. Palgic, Arbinoxa) • Optivar (Azelastine) oChlorpheniramine (ex. Actifed, Aller-chlor, Chlor- • Pataday, Patanol (Olopatadine) Trimeton, Tylenol Allergy) oDimenhydrinate (ex. Dramamine) HEARTBURN MEDICATIONS (H2 BLOCKERS): oDiphenhydramine (ex. Unisom, Sominex, • Axid (Nizatidine) Triaminic, many with “PM” in the title) • Pepcid, Tums Dual Action (Famotidine) oDoxylamine (ex. Nyquil, Unisom) • Tagament -

Orodispersible Tablets Im Mund Dispergierbare Tabletten Comprimés Orodispersibles
(19) TZZ Z_T (11) EP 2 572 705 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/00 (2006.01) A61K 9/20 (2006.01) 13.09.2017 Bulletin 2017/37 A61K 31/551 (2006.01) A61K 31/422 (2006.01) A61K 31/4178 (2006.01) (21) Application number: 12198042.9 (22) Date of filing: 30.09.2008 (54) Orodispersible tablets Im Mund dispergierbare Tabletten Comprimés orodispersibles (84) Designated Contracting States: • Díez Martín, Ignacio AT BE BG CH CY CZ DE DK EE ES FI FR GB GR E-08980 Sant Feliu de Llobregat - Barcelona (ES) HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT • Pablo Alba, Pablo RO SE SI SK TR E-08940 Cornella de Llobregat - Barcelona (ES) (30) Priority: 01.10.2007 EP 07380265 (74) Representative: ABG Patentes, S.L. 03.10.2007 US 977166 P Avenida de Burgos, 16D Edificio Euromor (43) Date of publication of application: 28036 Madrid (ES) 27.03.2013 Bulletin 2013/13 (56) References cited: (62) Document number(s) of the earlier application(s) in EP-A- 1 488 811 EP-A- 1 681 048 accordance with Art. 76 EPC: WO-A1-03/045844 WO-A2-2004/105680 08804908.5 / 2 205 213 DE-A1-102005 009 241 (73) Proprietor: Laboratorios Lesvi, S.L. • "Orally disintegrating pharmaceutical 08970 Sant Joan Despí - Barcelona (ES) composition", IP.COM JOURNAL, IP.COM INC., WEST HENRIETTA, NY, US, 11 September 2006 (72) Inventors: (2006-09-11), XP013115762, ISSN: 1533-0001 • Úbeda Pérez, Carmen E-08348 Cabrils - Barcelona (ES) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Antihistamines in the Treatment of Chronic Urticaria I Jáuregui,1 M Ferrer,2 J Montoro,3 I Dávila,4 J Bartra,5 a Del Cuvillo,6 J Mullol,7 J Sastre,8 a Valero5
Antihistamines in the treatment of chronic urticaria I Jáuregui,1 M Ferrer,2 J Montoro,3 I Dávila,4 J Bartra,5 A del Cuvillo,6 J Mullol,7 J Sastre,8 A Valero5 1 Service of Allergy, Hospital de Basurto, Bilbao, Spain 2 Department of Allergology, Clínica Universitaria de Navarra, Pamplona, Spain 3 Allergy Unit, Hospital La Plana, Villarreal (Castellón), Spain 4 Service of Immunoallergy, Hospital Clínico, Salamanca, Spain 5 Allergy Unit, Service of Pneumology and Respiratory Allergy, Hospital Clínic (ICT), Barcelona, Spain 6 Clínica Dr. Lobatón, Cádiz, Spain 7 Rhinology Unit, ENT Service (ICEMEQ), Hospital Clínic, Barcelona, Spain 8 Service of Allergy, Fundación Jiménez Díaz, Madrid, Spain ■ Summary Chronic urticaria is highly prevalent in the general population, and while there are multiple treatments for the disorder, the results obtained are not completely satisfactory. The second-generation H1 antihistamines remain the symptomatic treatment option of choice. Depending on the different pharmacokinetics and H1 receptor affi nity of each drug substance, different concentrations in skin can be expected, together with different effi cacy in relation to the histamine-induced wheal inhibition test - though this does not necessarily have repercussions upon clinical response. The antiinfl ammatory properties of the H1 antihistamines could be of relevance in chronic urticaria, though it is not clear to what degree they infl uence the fi nal therapeutic result. Before moving on to another therapeutic level, the advisability of antihistamine dose escalation should be considered, involving increments even above those approved in the Summary of Product Characteristics. Physical urticaria, when manifesting isolatedly, tends to respond well to H1 antihistamines, with the exception of genuine solar urticaria and delayed pressure urticaria.