Chemical Weathering in Highsedimentyielding Watersheds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Full Article
NOTORNIS QUARTERLY JOURNAL of the Ornithological Society of New Zealand Volume Sixteen, Number Two, lune, 1969 NOTICE TO CONTRIBUTORS Contributions should be type-written, double- or treble-spaced, with a wide margin, on one side of the paper only. They should be addressed to the Editor, and are accepted o?, condition that sole publication is being offered in the first instance to Notornis." They should be concise, avoid repetition of facts already published, and should take full account of previous literature on the subject matter. The use of an appendix is recommended in certain cases where details and tables are preferably transferred out of the text. Long contributions should be provided with a brief summary at the start. Reprints: Twenty-five off-prints will be supplied free to authors, other than of Short Notes. When additional copies are required, these will be produced as reprints, and the whole number will be charged to the author by the printers. Arrangements for such reprints must be made directly between the author and the printers, Te Rau Press Ltd., P.O. Box 195, Gisborne, prior to publication. Tables: Lengthy and/or intricate tables will usually be reproduced photographically, so that every care should be taken that copy is correct in the first instance. The necessity to produce a second photographic plate could delay publication, and the author may be called upon to meet the additional cost. nlastrutions: Diagrams, etc., should be in Indian ink, preferably on tracing cloth, and the lines and lettering must be sufficiently bold to allow of reduction. Photographs must be suitable in shape to allow of reduction to 7" x 4", or 4" x 3f". -

Ïg8g - 1Gg0 ISSN 0113-2S04
MAF $outtr lsland *nanga spawning sur\feys, ïg8g - 1gg0 ISSN 0113-2s04 New Zealand tr'reshwater Fisheries Report No. 133 South Island inanga spawning surv€ys, 1988 - 1990 by M.J. Taylor A.R. Buckland* G.R. Kelly * Department of Conservation hivate Bag Hokitika Report to: Department of Conservation Freshwater Fisheries Centre MAF Fisheries Christchurch Servicing freshwater fisheries and aquaculture March L992 NEW ZEALAND F'RESTTWATER F'ISHERIES RBPORTS This report is one of a series issued by the Freshwater Fisheries Centre, MAF Fisheries. The series is issued under the following criteria: (1) Copies are issued free only to organisations which have commissioned the investigation reported on. They will be issued to other organisations on request. A schedule of reports and their costs is available from the librarian. (2) Organisations may apply to the librarian to be put on the mailing list to receive all reports as they are published. An invoice will be sent for each new publication. ., rsBN o-417-O8ffi4-7 Edited by: S.F. Davis The studies documented in this report have been funded by the Department of Conservation. MINISTBY OF AGRICULTUBE AND FISHERIES TE MANAlU AHUWHENUA AHUMOANA MAF Fisheries is the fisheries business group of the New Zealand Ministry of Agriculture and Fisheries. The name MAF Fisheries was formalised on I November 1989 and replaces MAFFish, which was established on 1 April 1987. It combines the functions of the t-ormer Fisheries Research and Fisheries Management Divisions, and the fisheries functions of the former Economics Division. T\e New Zealand Freshwater Fisheries Report series continues the New Zealand Ministry of Agriculture and Fisheries, Fisheries Environmental Report series. -

May 2019, Issue 4
THE NEWS MAY 2019 S ISSUE 5 REDUCTION READINESS RESPONSE RECOVERY IN THIS ISSUE > Westland bears the brunt of heavy rainfall > AF8 Roadshow > Understanding the risk > New Zealand ShakeOut 2019 > Kid's time! Welcome to our Autumn newsletter! Looking after yourself and your family in an emergency | Readiness plans Many disasters will affect A household emergency plan will help you work out: essential services and possibly n What you will each do in the event n How and when to turn off the water, disrupt your ability to travel or of disasters such as an earthquake, electricity and gas at the main communicate with each other. tsunami, volcanic eruption, flood or switches in your home or business. storm. You may be confined to your home or Turn off gas only if you suspect a n How and where you will meet up during forced to evacuate your neighbourhood. leak, or if you are instructed to do so and after a disaster. In the immediate aftermath of a disaster, by authorities. If you turn the gas off emergency services may not be able to get n Where to store emergency survival you will need a professional to turn it help to everyone as quickly as needed. items and who will be responsible for back on and it may take them weeks to maintaining supplies. This is when you are likely to be most respond after an event. n vulnerable, so it is important to plan to What you will each need to have in your n What local radio stations to tune in to look after yourself and your loved ones getaway kits and where to keep them. -

Haast Regional Walks Brochure
Mäori first settled here at least 800 years ago, the sea, Haast Visitor Centre Introduction coast and navigable rivers providing main points of access. Mäori settlement and activity was centred around Information on the Te Wähipounamu - South West New The Haast area is more than a collection of small gathering, carving and trading precious jade, known as Zealand World Heritage Area, other lands administered by settlements near the main highway or along the road to pounamu (greenstone). Jackson Bay Okahu. It is a diverse region, stretching the Department of Conservation, tracks, accommodation European settlement was attempted at Jackson Bay Okahu from Knights Point to the Cascade Valley and inland to the and advice on recreational opportunities in the Haast area during the 1870s. The pioneers’ attempt to “tame” the forest-lined Haast Pass. The area offers a wide variety of can be obtained from the Haast Visitor Centre at Haast landscape was largely unsuccessful but their efforts left scenery, chances to view wildlife and many recreational (situated on the corner of SH6 and the Jackson Bay Road). a tradition of South Westland residents as being tough, opportunities. Hut tickets, hunting permits, maps, conservation souvenirs resilient and independent. and publications can also be obtained from the visitor The region is famous for it’s dramatic coastline - the This brochure should help visitors find their way around the centre. EFTPOS is available. sweeping curves of beaches, the rugged cliff tops, and Haast area. Displays at the Department of Conservation’s the striking rock formations at Knights Point south of Lake Haast Visitor Centre and at other sites within the World Moeraki. -

Waiho River Flooding Risk Assessment MCDEM 2002.Pdf
Report 80295/2 Page i WAIHO RIVER FLOODING RISK ASSESSMENT For MINISTRY OF CIVIL DEFENCE & EMERGENCY MANAGEMENT AUGUST 2002 Report 80295/2 Page ii PROJECT CONTROL Consultant Details Project Leader: Adam Milligan Phone: 04 471 2733 Project Team: Fax: 04 471 2734 Organisation: Optimx Limited Postal Address: P O Box 942, Wellington Peer Reviewer Details Reviewer: David Elms Phone: 03 364 2379 Position: Director Fax: 03 364 2758 Organisation: Optimx Limited Postal Address: Department of Civil Engineering University of Canterbury Private Bag 4800, Christchurch Client Details Client: Ministry of Civil Defence & Emergency Postal Address: PO Box 5010 Management Wellington Client contact: Richard O’Reilly Phone: 04 473 7363 Project Details Project: Waiho River Flooding Risk Assessment Project No.: 80295 Version Status File Date Approval signatory 1 Draft 80295 MCDEM Waiho River Risk Assessment Report - Aug 2002 David Elms Version 1.doc 2 Final Draft 80295 MCDEM Waiho River Risk Assessment Report - Aug 2002 David Elms Version 2.doc 3 Final 80295 MCDEM Waiho River Risk Assessment Report - Aug 2002 David Elms FINAL.doc This report has been prepared at the specific instructions of the Client. Only the Client is entitled to rely upon this report, and then only for the purpose stated. Optimx Limited accept no liability to anyone other than the Client in any way in relation to this report and the content of it and any direct or indirect effect this report my have. Optimx Limited does not contemplate anyone else relying on this report or that it will be used for any other purpose. Should anyone wish to discuss the content of this report with Optimx Limited, they are welcome to contact the project leader at the address stated above. -

Table of Contents. Letter of Transmittal. Officers 1910
TWELFTH REPORT OFFICERS 1910-1911. OF President, F. G. NOVY, Ann Arbor. THE MICHIGAN ACADEMY OF SCIENCE Secretary-Treasurer, GEO. D. SHAFER, East Lansing. Librarian, A. G. RUTHVEN, Ann Arbor. CONTAINING AN ACCOUNT OF THE ANNUAL MEETING VICE-PRESIDENTS. HELD AT Agriculture, CHARLES E. MARSHALL, East Lansing. Geography and Geology, W. H. SHERZER, Ypsilanti. ANN ARBOR, MARCH 31, APRIL 1 AND 2, 1910. Zoology, A. S. PEARSE, Ann Arbor. Botany, C. H. KAUFFMAN, Ann Arbor. PREPARED UNDER THE DIRECTION OF THE Sanitary and Medical Science, GUY KIEFER, Detroit. COUNCIL Economics, H. S. SMALLEY, Ann Arbor. BY PAST-PRESIDENTS. GEO. D. SHAFER DR. W. J. BEAL, East Lansing. Professor W. H. SHERZER, Ypsilanti. BRYANT WALKER, ESQ. Detroit. BY AUTHORITY Professor V. M. SPALDING, Tucson, Arizona. LANSING, MICHIGAN DR. HENRY B. BAKER, Holland. WYNKOOP HALLENBECK CRAWFORD CO., STATE PRINTERS Professor JACOB REIGHARD, Ann Arbor. 1910 Professor CHARLES E. BARR, Albion. Professor V. C. VAUGHAN, Ann Arbor. Professor F. C. NEWCOMBE, Ann Arbor. TABLE OF CONTENTS. DR. A. C. LANE, Tuft's College, Mass. Professor W. B. BARROWS, East Lansing. DR. J. B. POLLOCK, Ann Arbor. Letter of Transmittal .......................................................... 1 Professor M. H. W. JEFFERSON, Ypsilanti. DR. CHARLES E. MARSHALL, East Lansing. Officers for 1910-1911. ..................................................... 1 Professor FRANK LEVERETT, Ann Arbor. Life of William Smith Sayer. .............................................. 1 COUNCIL. Life of Charles Fay Wheeler.............................................. 2 The Council is composed of the above named officers Papers published in this report: and all Resident Past-Presidents. President's Address—Outline of the History of the Great Lakes, Frank Leverett.......................................... 3 On the Glacial Origin of the Huronian Rocks of WILLIAM SMITH SAYER. -

NEW ZEALAND NOTES·, 1954-5 by DAVID HALL
• NEW ZEALAND NOTES, 1954-5 39 1 . NEW ZEALAND NOTES·, 1954-5 By DAVID HALL HE last climbing season was notable for two things : first, the early disappearance of snow providing in early January conditions which might have been expected six weeks later ; second, the high death roll in accidents. A consequence of the lack of snow was that much rock was exposed which would have been concealed at a normal Christmas season and many slopes were uncompromisingly hard ice which would normally · have been steep, firm snow; everyone in fact had to work fairly hard for the climbs they did. But some very good climbs were done. The existence (or should one say the ' invention ') of new ten thousanders must be a perpetual menace to the peace of mind of those few choice spirits who have achieved what they hoped was an unfading garland with the ascent of all the peaks in New Zealand over 1o,ooo ft. However, Mount ' Magellan ' is not positively claimed as definitely 1o,ooo ft., although it must closely approximate to it. (Throughout these notes names cited in inverted commas have yet to receive the approval of the Geographic Board.) ' Magellan ' lies on the Balfour Range spur of Mount Teichelmann, and its first ascent was made in February by Guide Harry Ayres with B. S. Gillies and Sir Edmund Hillary and D. G. Herron; the two parties climbed some of the day on one rope. They ieft the Haast Hut at 2 A.M., were· at the summit of Teichelmann by way of Clarke Saddle by 10.45 A.M., reached their objective, after traversing a long teetery crest of snow interrupted by a formidable rock tower, at 4 P.M., and returned to the Haast Hut by the route of ascent twenty-four hours after setting forth. -

Open Kosei.Pdf
The Pennsylvania State University The Graduate School Department of Geosciences GEOCHEMISTRY OF ARCHEAN–PALEOPROTEROZOIC BLACK SHALES: THE EARLY EVOLUTION OF THE ATMOSPHERE, OCEANS, AND BIOSPHERE A Thesis in Geosciences by Kosei Yamaguchi Copyright 2002 Kosei Yamaguchi Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy May 2002 We approve the thesis of Kosei Yamaguchi Date of Signature ____________________________________ _______________________ Hiroshi Ohmoto Professor of Geochemistry Thesis Advisor Chair of Committee ____________________________________ _______________________ Michael A. Arthur Professor of Geosciences ____________________________________ _______________________ Lee R. Kump Professor of Geosciences ____________________________________ _______________________ Raymond G. Najjar Associate Professor of Meteorology ____________________________________ _______________________ Peter Deines Professor of Geochemistry Associate Head for Graduate Program and Research in Geosciences iii ABSTRACT When did the Earth's surface environment become oxic? The timing and mechanism of the rise of atmospheric pO2 level in the early Precambrian have been long debated but no consensus has been reached. The oxygenation of the atmosphere and oceans has significant impacts on the evolution of the biosphere and the geochemical cycles of redox-sensitive elements. In order to constrain the evolution of the atmosphere, oceans, biosphere, and geochemical cycles of elements, a systematic and multidisciplinary -

Scanned Using Fujitsu 6670 Scanner and Scandall Pro Ver 1.7 Software
1617 1980/226 THE FRESHWATER FISHERIES REGULATIONS (WEST COAST AND WESTLAND) MODIFICATION NOTICE 1980 PURSUANT to section 83 (2) ( d) of the Fisheries Act 1908, and to regulation 7 of the Freshwater Fisheries Regulations 1951, the Minister of Agriculture and Fisheries hereby gives the following notice. NOTICE 1. Title-This notice may be cited as the Freshwater Fisheries Regulations (West Coast and Westland) Modification Notice 1980. 2. Commencement-This notice shall come into force on the 14th day after the date of its notification in the Gazette. 3. Application-This notice shall be in force only within the West Coast and Westland Acclimatisation Districts. 4. Modification of regulations-The Freshwater Fisheries Regulations 1951 * are hereby modified as follows: Limit Bag (a ) No person shall on anyone day take or kill more than 14 acclimatised fish (being trout or salmon) of which no more than 4 may be salmon and no more than 10 may be trout: Size Limit (b) No person shall take or kill in any manner whatever or inten tionally have in his possession any trout or salmon that does not exceed- (i) In the case of any salmon, 30 cm in length: (ii) In the case of any trout, 25 cm in length: Open Season Exceptions (c) No person shall fish at any time for acclimatised fish in any stream flowing into Lake Wahapo or Lake Mapourika: *S.R. 1951/15 (Reprinted with Amendments Nos. 1 to 13: S.R. 1976/191) Amendment No. 14: (Revoked by S.R. 19761268) Amendment No. 15: S.R. 19761268 Amendment No. -

Supplement 9: Regional Flood Control Assets
West Coast Lifelines Vulnerability and Interdependency Assessment Supplement 9: Regional Flood Control Assets West Coast Civil Defence Emergency Management Group August 2017 IMPORTANT NOTES Disclaimer The information collected and presented in this report and accompanying documents by the Consultants and supplied to West Coast Civil Defence Emergency Management Group is accurate to the best of the knowledge and belief of the Consultants acting on behalf of West Coast Civil Defence Emergency Management Group. While the Consultants have exercised all reasonable skill and care in the preparation of information in this report, neither the Consultants nor West Coast Civil Defence Emergency Management Group accept any liability in contract, tort or otherwise for any loss, damage, injury or expense, whether direct, indirect or consequential, arising out of the provision of information in this report. This report has been prepared on behalf of West Coast Civil Defence Emergency Management Group by: Ian McCahon BE (Civil), David Elms BA, MSE, PhD Rob Dewhirst BE, ME (Civil) Geotech Consulting Ltd 21 Victoria Park Road Rob Dewhirst Consulting Ltd 29 Norwood Street Christchurch 38A Penruddock Rise Christchurch Westmorland Christchurch Hazard Maps The hazard maps contained in this report are regional in scope and detail, and should not be considered as a substitute for site-specific investigations and/or geotechnical engineering assessments for any project. Qualified and experienced practitioners should assess the site-specific hazard potential, including the potential for damage, at a more detailed scale. Cover Photo: Greymouth Floodwall, Grey River, Greymouth West Coast Lifelines Vulnerability and Interdependency Assessment Supplement 9: Regional Flood Control Assets Contents 1 INTRODUCTION ........................................................................................................................ -
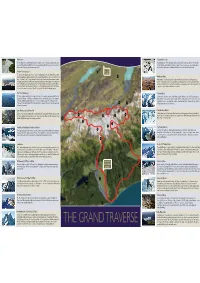
Franz Josef Welcome Aboard ENGLISH Copy
1 Waiho River 11 Tasman Glacier Lake This incredibly active silt-laden river drains the melting ice from the Franz Josef Glacier and runoff This lake formed in 1991 and has been growing ever since. The icebergs, which are clearly visible from the Callery Valley. The Waiho River has been aggrading at 300 mm/yr in recent times and is at from the air, have taken about 500 years to travel from the neve at the top of the Tasman Glacier present some 2 metres above the surrounding land. to where they appear today. Underneath this lake the ice is still over 200 metres thick. AIR SAFARIS LAKE Okarito Forest & Lagoon LAKE TEKAPO TEKAPO AIRPORT The ancient dense Okarito rainforest is home to a small population of the rare Rowi (Okarito brown LAKE 2 Kiwi). The population is under considerable threat from introduced animals such as rats and stoats that 12 PUKAKI 12 Mackenzie Basin prey on the kiwi. To the North you can see the Okarito Lagoon, famous as a bird watchers’ paradise. Approximately 14,000 years ago the ice that covered this area from the last Ice Age began its Thousands of native birds, including nearly every mainland species in New Zealand, visit or make their retreat – today golden tussock and grasslands cover the glacial deposits that remain clearly visible home on this lagoon. It is perhaps best known for the kotuku (white heron) which breed here. These from the air. Dramatic ice-carved landscape, subtle ever-changing hues, and air of exceptional purity are a sacred bird to the Maori people. -

Sedimentology, Petrography, and Depositional Environment of Dore
SEDIMENTOLOGY, PETROGRAPHY, AND DEPOSITIONAL ENVIRONMENT OF DORE SEDIMENTS ABOVE THE HELEN-ELEANOR IRON RANGE, WAHA, ONTARIO ' SEDIMENTOLOGY, PETROGRAPHY, AND DEPOSITIONAL ENVIRONMENT OF DORE SEDIMENTS AHOVE THE HELEN-ELEANOR IRON RANGE, WAWA, ONTARIO By KATHRYN L. NEALE Submitted to the Department of Geology in Partial Fulfilment of the Requirements for the Degree Bachelor of Science McMaster University April, 1981 BACHELOR OF SCIENCE (1981) McMASTER UNIVERSITY (Geology) Hamilton, Ontario TITLE: Sedimentology, Petrography, and Depositional Environment of Dore Sediments above the Helen Eleanor Iron Range, Wawa, Ontario AUTHOR: Kathryn L. Neale SUPERVISOR: Dr. R. G. Walker NUMBER OF PAGES: ix, 54 ii ABSTRACT Archean sediments of the Dare group, located in the 1-Jawa green stone belt, were studied. The sediments are stratigraphically above the carbonate facies Helen-Eleanor secti on of the Michipicoten Iron Forma tion. A mafic volcanic unit, 90 m thick, lies between the iron range and the sediments. Four main facies have been i dentified in the first cycle of clastic sedimentation above the maf i c flow rocks. A 170 ro break in stratigraphy separates the volcanics from the first facies. The basal sedimentary facies is an unstratified and poorly sorted granule-cobble conglomerate, 200m thick, interpreted as an alluvial mass flow deposit. Above the conglomerate, there is a 20 m break in the stratigraphic column. The second facies, 220m to 415 m thick, consists of laminated (0.1- 2 em) argillites and siltstones, together with massive, thick bedded (8-10 m) greywackes. The argillites and siltstones are interpre ted as interchannel deposits on an upper submarine fan, and the grey wackes are explained as in-channel turbidity current deposition.