A Multi-Locus Inference of the Evolutionary Diversification of Extant Flamingos (Phoenicopteridae) Torres Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

6-A John James Audubon, American Flamingo, 1838
JOHN JAMES AUDUBON [1785–1851] 6 a American Flamingo,1838 American Flamingo is one of the 435 hand-colored engravings that River, a major flyway for migratory birds, and eventually wan- make up John James Audubon’s monumental Birds of America, dered farther from home to comb the American frontier for issued in four volumes between 1826 and 1838. The massive unrecorded species. publication includes life-size representations of nearly five hundred Audubon’s procedure was to study and sketch a bird in its natural species of North American birds. Although Audubon was not the habitat before killing it carefully, using fine shot to minimize dam- first to attempt such a comprehensive catalog, his work departed age. His critical innovation was to then thread wire through the from conventional scientific illustration, which showed lifeless spec- specimen, allowing him to fashion a lifelike pose. He worked in imens against a blank background, by presenting the birds as they watercolor, and had completed some four hundred paintings appeared in the wild. When his pictures were first published, when he decided to publish them as a folio of prints. Failing to find some naturalists objected to Audubon’s use of dramatic action and support in Philadelphia, he sailed for England, where he became pictorial design, but these are the qualities that set his work apart lionized as “The American Woodsman.” The engraving firm and make it not only an invaluable record of early American Robert Havell and Son took on the challenge of reproducing wildlife but an unmatched work of American art. Audubon’s paintings on copper plates and tinting the resulting John James Audubon was born in Haiti and educated in France, black-and-white prints by hand. -

A Pliocene Flamingo from Mexico
June, 1944 THE WILSON BULLETIN 77 Vol. 56. No. 2 A PLIOCENE FLAMINGO FROM MEXICO BY LOYE MILLER IELD parties from the California Institute of Technology have been F fortunate in locating a variety of fossil deposits in Mexico that in- cluded bird remains. Some have been very rich in the quantity and variety of material; for example, the San Josecito Cavern of Nuevo Leon (Miller, ,1943), a deposit of Pleistocene age, yielded several thousand bird bones assigned to over forty species. The present paper deals with a collection of ten fragments, all but one of which are in- cluded in a single species. I am indebted to Dr. Chester Stock in charge of the explorations for the opportunity of working with the bird collec- tions. Dr. Alexander Wetmore has loaned comparative material, and Dr. Hildegarde Howard has been a most congenial fellow student during many conferences on the flamingoes, both Recent and Fossil. To these several colleagues my sincere thanks are offered. The ten fragments are from collecting locality No. 289, California Institute of Technology, known. as the Rincon Pliocene, Chihuahua, Mexico. Associated mammal remains include horse, camel, antelope, and carnivore species. The matrix is a fine grained silt of lightest color, without cementing material. A stiff brush serves to remove it from the well petrified bones. Unfortunately the specimens are most frag- mentary. They do, however, prove to be of interest in several respects; most notably they prove (since several speciments are from pre-volant young) that a small speciesof flamingo was present as a breeding bird. This is the earliest record for the family in America. -

The Tarsometatarsus of the Middle Eocene Loon Colymbiculus Udovichenkoi
– 17 – Paleornithological Research 2013 Proceed. 8th Inter nat. Meeting Society of Avian Paleontology and Evolution Ursula B. Göhlich & Andreas Kroh (Eds) The tarsometatarsus of the Middle Eocene loon Colymbiculus udovichenkoi GERALD MAYR1, LEONID GOROBETS2 & EVGENIJ ZVONOK3 1 Senckenberg Research Institute and Natural History Museum Frankfurt, Frankfurt am Main, Germany; E-mail: [email protected] 2 Taras Shevchenko National University of Kiev, Dept. of Ecology and Environmental Protection, Kiev, Ukraine 3 Institute of Geological Sciences of NAS of Ukraine, Branch of Paleontology and Stratigraphy, Kiev, Ukraine Abstract — We describe the previously unknown tarsometatarsus of the earliest unambiguously identified loon, Colymbiculus udovichenkoi, from the Middle Eocene of the Ukraine. Except for being more elongate and apart from details of the hypotarsus morphology, the bone resembles the tarsometatarsus of the Early Miocene Colym- boides minutus. We consider the hypotarsus morphology of Colymbiculus to be plesiomorphic for Gaviiformes. Colymboides and crown group Gaviiformes are each characterized by an autapomorphic hypotarsus morphology, which precludes the former from being directly ancestral to the latter. The similarities shared by C. udovichenkoi and C. minutus, including their small size, are likely to be plesiomorphic for Gaviiformes. Although the disap- pearance of small stem group Gaviiformes may be related to the retreat of loons to cold Northern latitudes, more data are needed to firmly establish this hypothesis. We finally note that early Paleogene stem group Gaviiformes markedly differ from putative Late Cretaceous loons, whose identification needs to be verified by further fossil specimens. Key words: Colymbiculus, Colymboides, fossil birds, Gaviiformes, Eocene, Lutetian, Ukraine Introduction 1982 from the Early Miocene of the Czech Republic (ŠVEC 1982), the earliest stem line- Loons (Gaviiformes) have a fairly comprehen- age representative, being distinctly smaller than sive Neogene fossil record (OLSON 1985; MAYR its modern congeners. -

Flamingo ABOUT the GROUP
Flamingo ABOUT THE GROUP Bulletin of the IUCN-SSC/Wetlands International The Flamingo Specialist Group (FSG) was established in 1978 at Tour du Valat in France, under the leadership of Dr. Alan Johnson, who coordinated the group until 2004 (see profile at www.wetlands.org/networks/Profiles/January.htm). Currently, the group is FLAMINGO SPECIALIST GROUP coordinated from the Wildfowl & Wetlands Trust at Slimbridge, UK, as part of the IUCN- SSC/Wetlands International Waterbird Network. The FSG is a global network of flamingo specialists (both scientists and non- scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research and conservation worldwide by encouraging information exchange and cooperation amongst these specialists, and with other relevant organisations, particularly IUCN - SSC, Ramsar, WWF International and BirdLife International. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from field surveys to breeding biology, diseases, tracking movements and data management. There are currently 165 members around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Assistant Chair Dr. Brooks Childress Mr. Nigel Jarrett Wildfowl & Wetlands Trust Wildfowl & Wetlands Trust Slimbridge Slimbridge Glos. GL2 7BT, UK Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Tel: +44 (0)1453 891177 Fax: +44 (0)1453 860437 Fax: +44 (0)1453 890827 [email protected] [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. -
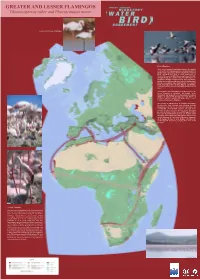
GREATER and LESSER FLAMINGOS Phoenicopterus Ruber and Phoeniconaias Minor
GREATER AND LESSER FLAMINGOS Phoenicopterus ruber and Phoeniconaias minor Greater and Lesser Flamingos © Cliff Buckton © P & H Harris Lesser Flamingo The Lesser Flamingo Phoeniconaias minor is the smallest of the world's five flamingo species. It occurs primarily in the Rift Valley lakes of East Africa with about 4 to 5 million birds estimated, but also in small populations in Namibia/Botswana (40,000), Mauritania/Senegal (15,400), Ethiopia (8,300). The alkaline lakes of the Rift Valley are the primary feeding areas for the East Africa population. During non-breeding periods these lakes often hold almost the entire population. Huge feeding flocks of 1-2 million birds frequently gather on lakes Bogoria and Nakuru, creating one of the most stunning wildlife spectacles in the world. Although it is still the most numerous of the five species, the Lesser Flamingo is classified as globally "near threatened" due primarily to its dependence on a limited number of unprotected breeding sites and threats of proposed soda-ash mining and hydro-electric power schemes on the main breeding lakes. The question of whether there is occasional interchange between the East African and southern African populations has yet to be resolved definitely, but considerable circumstantial evidence has now been assembled to show that East African Lesser Flamingos probably do fly to Botswana to breed during periods when the Lake Makgadikgadi Salt Pans are flooded. Their migration routes, flight range and stopover places (if any) are still unknown. It is now known that Lesser Flamingos do fly during the day, at great heights, well above the normal diurnal movement of eagles, their main aerial predator. -

Dieter Thomas Tietze Editor How They Arise, Modify and Vanish
Fascinating Life Sciences Dieter Thomas Tietze Editor Bird Species How They Arise, Modify and Vanish Fascinating Life Sciences This interdisciplinary series brings together the most essential and captivating topics in the life sciences. They range from the plant sciences to zoology, from the microbiome to macrobiome, and from basic biology to biotechnology. The series not only highlights fascinating research; it also discusses major challenges associated with the life sciences and related disciplines and outlines future research directions. Individual volumes provide in-depth information, are richly illustrated with photographs, illustrations, and maps, and feature suggestions for further reading or glossaries where appropriate. Interested researchers in all areas of the life sciences, as well as biology enthusiasts, will find the series’ interdisciplinary focus and highly readable volumes especially appealing. More information about this series at http://www.springer.com/series/15408 Dieter Thomas Tietze Editor Bird Species How They Arise, Modify and Vanish Editor Dieter Thomas Tietze Natural History Museum Basel Basel, Switzerland ISSN 2509-6745 ISSN 2509-6753 (electronic) Fascinating Life Sciences ISBN 978-3-319-91688-0 ISBN 978-3-319-91689-7 (eBook) https://doi.org/10.1007/978-3-319-91689-7 Library of Congress Control Number: 2018948152 © The Editor(s) (if applicable) and The Author(s) 2018. This book is an open access publication. Open Access This book is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. -

Ichnotaxonomy of the Eocene Green River Formation
Ichnotaxonomy of the Eocene Green River Formation, Soldier Summit and Spanish Fork Canyon, Uinta Basin, Utah: Interpreting behaviors, lifestyles, and erecting the Cochlichnus Ichnofacies By © 2018 Joshua D. Hogue B.S. Old Dominion University, 2013 Submitted to the graduate degree program in Geology and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Master of Science. Chair: Dr. Stephen T. Hasiotis Dr. Paul Selden Dr. Georgios Tsoflias Date Defended: May 1, 2018 ii The thesis committee for Joshua D. Hogue certifies that this is the approved version of the following thesis: Ichnotaxonomy of the Eocene Green River Formation, Soldier Summit and Spanish Fork Canyon, Uinta Basin, Utah: Interpreting behaviors, lifestyles, and erecting the Cochlichnus Ichnofacies Chair: Dr. Stephen T. Hasiotis Date Approved: May 1, 2018 iii ABSTRACT The Eocene Green River Formation in the Uinta Basin, Utah, has a diverse ichnofauna. Nineteen ichnogenera and 26 ichnospecies were identified: Acanthichnus cursorius, Alaripeda lofgreni, c.f. Aquatilavipes isp., Aulichnites (A. parkerensis and A. tsouloufeidos isp. nov.), Aviadactyla (c.f. Av. isp. and Av. vialovi), Avipeda phoenix, Cochlichnus (C. anguineus and C. plegmaeidos isp. nov.), Conichnus conichnus, Fuscinapeda texana, Glaciichnium liebegastensis, Glaroseidosichnus ign. nov. gierlowskii isp. nov., Gruipeda (G. fuenzalidae and G. gryponyx), Midorikawapeda ign. nov. semipalmatus isp. nov., Planolites montanus, Presbyorniformipes feduccii, Protovirgularia dichotoma, Sagittichnus linki, Treptichnus (T. bifurcus, T. pedum, and T. vagans), and Tsalavoutichnus ign. nov. (Ts. ericksonii isp. nov. and Ts. leptomonopati isp. nov.). Four ichnocoenoses are represented by the ichnofossils—Cochlichnus, Conichnus, Presbyorniformipes, and Treptichnus—representing dwelling, feeding, grazing, locomotion, predation, pupation, and resting behaviors of organisms in environments at and around the sediment-water-air interface. -

Fossil Birds of the Nebraska Region
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Transactions of the Nebraska Academy of Sciences and Affiliated Societies Nebraska Academy of Sciences 1992 Fossil Birds of the Nebraska Region James E. Ducey [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/tnas Part of the Life Sciences Commons Ducey, James E., "Fossil Birds of the Nebraska Region" (1992). Transactions of the Nebraska Academy of Sciences and Affiliated Societies. 130. https://digitalcommons.unl.edu/tnas/130 This Article is brought to you for free and open access by the Nebraska Academy of Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Transactions of the Nebraska Academy of Sciences and Affiliated Societiesy b an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 1992. Transactions of the Nebraska Academy of Sciences, XIX: 83-96 FOSSIL BIRDS OF THE NEBRASKA REGION James Ducey 235 Nebraska Hall Lincoln, Nebraska 68588-0521 ABSTRACT Crane (Grus haydeni = Grus canadensis) (Marsh, 1870) and a species of hawk (Buteo dananus) from along the This review compiles published and a few unpublished Loup Fork (Marsh, 1871). records offossil and prehistoric birds for the Nebraska region (Nebraska and parts of adjacent states) from the Cretaceous Many ofthe species first described were from mate Period to the late Pleistocene, about 12,000 years before present. Species recorded during the various epochs include: rial collected in the Great Plains region, including Kan Oligocene and Early Miocene (13 families; 29 species), Middle sas and Wyoming (Marsh, 1872b). The work of scien Miocene (six families; 12 species), Late Miocene (14 families; tists associated with the University of Nebraska in 21 species), Pliocene (six families; 15 species), Early-Middle cluded studies made around the turn-of-the-century. -

Proceedings of the Ninety-First Stated Meeting of the American Ornithologists' Union
PROCEEDINGS OF THE NINETY-FIRST STATED MEETING OF THE AMERICAN ORNITHOLOGISTS' UNION RICHARDC. BANKS•SECRETARY TI-IE Ninety-first Stated Meeting of the American Ornithologists' Union was held 8-12 October 1973 at Provincetown, on Cape Cod, Massachusetts, under the sponsorshipof the Nuttall OrnithologicalClub, which was celebrating its centennialyear. Business,technical, and social sessionswere held in the ProvincetownInn. Field trips were taken to various localitieson Cape Cod. BUSINESS SESSIONS The Councilmet in the morningand afternoonof 8 Octoberand again in the afternoon of 10 October. The Fellows met in the late afternoon of 8 October and again in the afternoon of 11 October. Elective Members and Fellows met in the eveningof 8 October. A summaryof important actionsat these meetings follows: Future meetings.--The Ninety-secondStated Meeting will be held at the University of Oklahoma, Norman, Oklahoma, 14-18 October 1974, at the invitation of the University, the Oklahoma OrnithologicalSociety, and the ClevelandCounty Bird Club. The Ninety-third Stated Meeting will be held in Winnipeg, Manitoba, in August of 1975, at the invitation of the University of Manitoba. The Ninety-fourth Stated Meeting will take place at Hayerford College,near Philadelphia,Pennsylvania, in August 1976, at the invitation of the Academy of Natural Sciencesof Philadelphia and Hayerford College. An invitation for 1977 from the Museum of Vertebrate Zoology, University of California, Berkeley, was extended,as was one from the Linnean Society of New York for 1978. Action was not taken on these. Election o] oJJicers.--At the meeting of Elective Members and Fellows, Donald S. Farner was electedPresident; Harrison B. Tordoff was advancedto First Vice-President; Charles G. -

Avian Tree of Life Better Resolved 11 August 2020
Avian tree of life better resolved 11 August 2020 (Passeriformes), to which the species, genus and family rich songbirds belong. However, hardly any new families developed in the other bird orders. Manfred Gahr, Director at the Max Planck Institute for Ornithology, considers the invention of song learning as the driving force behind the diversity of songbirds: "With many songbirds, the song of the male determines the willingness of the female to mate. New songs may have contributed to the emergence of new species." Non-coding sequences The Max Planck team discovered that a certain molecular biological method can help to clearly The hoatzin had a last common ancestor with the differentiate bird groups. It is based on the analysis Caprimulgiformes (nightjar, sailors, hummingbirds) about of all active genes in a cell, the so-called 64 million years ago. Credit: Andre? Labetaa transcriptome. In addition to the sequences that are translated into proteins, the genes also contain various non-coding sequences. "These tend to be neglected. However, it has been shown that the Researchers led by Manfred Gahr of the Max non-coding sequences can resolve the avian family Planck Institute for Ornithology in Seewiesen have tree," says Heiner Kuhl, first author of the study, investigated the relationship of bird families. For who is now researching at the Leibniz Institute of the first time, they have been able to clarify the Freshwater Ecology and Inland Fisheries. relationship of all families of non-passerine birds and almost all families of passerine birds. The new "The non-coding sections contain sequences that family tree is based on gene sections that do not are typical for an entire bird family. -

Evidence for Aviculture: Identifying Research Needs to Advance the Role of Ex Situ Bird Populations in Conservation Initiatives and Collection Planning
Review Evidence for Aviculture: Identifying Research Needs to Advance the Role of Ex Situ Bird Populations in Conservation Initiatives and Collection Planning Paul Rose 1,2 1 Centre for Research in Animal Behaviour, Psychology, University of Exeter, Perry Road, Exeter, Devon EX4 4QG, UK; [email protected] or [email protected] 2 WWT, Slimbridge Wetland Centre, Slimbridge, Gloucestershire GL2 7BT, UK Simple Summary: Birds of a whole range of species are housed in zoological collections globally; they are some of the most frequently seen of species in animal populations kept under human care. Research output on birds can provide valuable information on how to advance husbandry and care for particular species, which may further feed into conservation planning. Linking birds housed in human care to those in the wild adds value to these zoo-housed populations; this paper provides areas of research that could be conducted to add value to these zoo-housed birds and suggests increasing the conservation focus and conservation relevance of birds housed by humans. Abstract: Birds are the most speciose of all taxonomic groups currently housed in zoos, but this species diversity is not always matched by their inclusion in research output in the peer-reviewed literature. This large and diverse captive population is an excellent tool for research investigation, the findings of which can be relevant to conservation and population sustainability aims. The One Plan Approach to conservation aims to foster tangible conservation relevance of ex situ populations to those animals living in situ. The use of birds in zoo aviculture as proxies for wild-dwelling Citation: Rose, P. -

The Renaissance of Avian Paleontology and Its Bearing on the Higher-Level Phylogeny of Birds
J Ornithol (2007) 148 (Suppl 2):S455–S458 DOI 10.1007/s10336-007-0159-8 REVIEW The renaissance of avian paleontology and its bearing on the higher-level phylogeny of birds Gerald Mayr Received: 12 February 2007 / Revised: 24 May 2007 / Accepted: 24 May 2007 / Published online: 20 June 2007 Ó Dt. Ornithologen-Gesellschaft e.V. 2007 Abstract Recent phylogenetic analyses provide strong by only a few specialists and, unlike the situation in evidence for some previously undetected clades of mor- mammalogy (e.g., Novacek 1992; Asher et al. 2003; phologically very divergent avian groups. Also, within the Boisserie et al. 2005), fossil taxa are usually not considered past decades, palaeornithology has experienced a renais- in analyses of and discussions on the phylogeny of extant sance and the Paleogene fossil record of birds is avian taxa, other than calibrations of molecular clocks approaching that of mammals in the number of recorded (e.g., Cracraft et al. 2004; Livezey and Zusi 2007). Livezey higher-level taxa. However, there is still little mutual ex- and Zusi (2007, p. 4) even went as far as to note that fossil change between students of these different data, as birds ‘‘typically provide only substandard anatomical molecular systematists are often unfamiliar with the fossil material or incomplete specimens’’. Certainly, this is not record of birds, whereas palaeornithologists only recently true. Many Paleogene taxa are known from complete started to interpret their fossils in the light of modern skeletons which are often complemented by three-dimen- phylogenetic analyses. Here, this deficiency is remedied in sionally preserved isolated bones, and sometimes even a brief overview of some fossil ‘‘missing links’’ between exhibit remarkable soft-tissue preservation (e.g., Mayr extant higher avian groups, which combine derived char- 2005a, 2006).