Basics of Pediatric Trauma Critical Care Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chest Trauma in Children
Chest Trauma In Children Donovan Dwyer MBBCh, DCH, DipPEC, FACEM Emergency Physician St George and Sydney Children’s Hospitals Director of Trauma, Sydney Children’s Hospital Disclaimer • This cannot be comprehensive • Trying to give trauma clinicians a perspective on paediatric differences • Trying to give emergency paediatric clinicians a perspective on trauma challenges • The format will focus on take home salient points related to ED dx and management • I may gloss over slides with more detail and references to keep to time • As most chest mortality is in the 12-15 yr age group, where paediatric evidence is low, I have looked to adult evidence OVERVIEW • Size of the problem • Common injuries – pitfalls and tips • Less common injuries – where recognition and time is critical • Blunt vs penetrating • The Chest in Traumatic Cardiac Arrest Size of the problem • Overall paediatric trauma in Australasia is low volume • Parents take injured children to nearest hospital for primary care • Prehospital services will divert to nearest facility with critically injured children Chest Injury • RV of Victorian State Trauma Registry in 2001–2007 = 204 cases • < 1/week • Blunt trauma - 96% • motor vehicle collisions (75%) • pedestrian (26%) • vehicle occupants (33%) Size of the problem • Common injuries – combined 50% of the time • lung contusion (66%) • haemo/pneumothorax (32%) • rib fracture (23%). • Associated multiple organ injury 90% • head (62%) • abdominal (50%) • Management conservative/supportive > 80% • Surgical • 11 cases (7%) treated surgically • 30% invasive • 18 cases(11%) had insertion of intercostal catheters (ICC). • Epidemiology of major paediatric chest trauma, Sumudu P Samarasekera ET AL, Journal of Paediatrics and Child Health (2009) International • 85 % Blunt –MVA – 45% • Ped vs car – 20% • Falls – 10% • NAI – 7% • Penetrating 15% • Mortality • Cooper A. -

Helping Young Children Who Have Experienced Trauma: Policies and Strategies for Early Care and Education
Helping Young Children Who Have Experienced Trauma: Policies and Strategies for Early Care and Education April 2017 Authors Acknowledgments Jessica Dym Bartlett, MSW, PhD We are grateful to our reviewers, Elizabeth Jordan, Senior Research Scientist Jason Lang, Robyn Lipkowitz, David Murphey, Child Welfare/Early Childhood Development Cindy Oser, and Kathryn Tout. We also thank Child Trends the Alliance for Early Success for its support of this work. Sheila Smith, PhD Director, Early Childhood National Center for Children in Poverty Mailman School of Public Health Columbia University Elizabeth Bringewatt, MSW, PhD Research Scientist Child Welfare Child Trends Copyright Child Trends 2017 | Publication # 2017-19 Helping Young Children Who Have Experienced Trauma: Policies and Strategies for Early Care and Education Table of Contents Executive Summary .............................................................1 Introduction ..........................................................................3 What is Early Childhood Trauma? ................................. 4 The Impacts of Early Childhood Trauma ......................5 Meeting the Needs of Young Children Who Have Experienced Trauma ...........................................................7 Putting It Together: Trauma-Informed Care for Young Children .....................................................................8 Promising Strategies for Meeting the Needs of Young Children Exposed to Trauma ..............................9 Recommendations ........................................................... -

DCMC Emergency Department Radiology Case of the Month
“DOCENDO DECIMUS” VOL 6 NO 2 February 2019 DCMC Emergency Department Radiology Case of the Month These cases have been removed of identifying information. These cases are intended for peer review and educational purposes only. Welcome to the DCMC Emergency Department Radiology Case of the Month! In conjunction with our Pediatric Radiology specialists from ARA, we hope you enjoy these monthly radiological highlights from the case files of the Emergency Department at DCMC. These cases are meant to highlight important chief complaints, cases, and radiology findings that we all encounter every day. PEM Fellowship Conference Schedule: February 2019 If you enjoy these reviews, we invite you to check out Pediatric Emergency Medicine Fellowship 6th - 9:00 First Year Fellow Presentations Radiology rounds, which are offered quarterly 13th - 8:00 Children w/ Special Healthcare Needs………….Dr Ruttan 9:00 Simulation: Neuro/Complex Medical Needs..Sim Faculty and are held with the outstanding support of the 20th - 9:00 Environmental Emergencies………Drs Remick & Munns Pediatric Radiology specialists at Austin Radiologic 10:00 Toxicology…………………………Drs Earp & Slubowski 11:00 Grand Rounds……………………………………….…..TBA Association. 12:00 ED Staff Meeting 27th - 9:00 M&M…………………………………..Drs Berg & Sivisankar If you have and questions or feedback regarding 10:00 Board Review: Trauma…………………………..Dr Singh 12:00 ECG Series…………………………………………….Dr Yee the Case of the Month, feel free to email Robert Vezzetti, MD at [email protected]. This Month: Let’s fall in love with learning from a very interesting patient. His story, though, is pretty amazing. Thanks to PEM Simulations are held at the Seton CEC. -
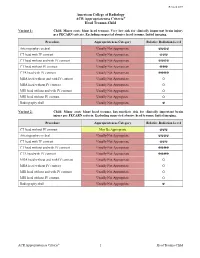
ACR Appropriateness Criteria: Head Trauma-Child
Revised 2019 American College of Radiology ACR Appropriateness Criteria® Head Trauma-Child Variant 1: Child. Minor acute blunt head trauma. Very low risk for clinically important brain injury per PECARN criteria. Excluding suspected abusive head trauma. Initial imaging. Procedure Appropriateness Category Relative Radiation Level Arteriography cerebral Usually Not Appropriate ☢☢☢☢ CT head with IV contrast Usually Not Appropriate ☢☢☢ CT head without and with IV contrast Usually Not Appropriate ☢☢☢☢ CT head without IV contrast Usually Not Appropriate ☢☢☢ CTA head with IV contrast Usually Not Appropriate ☢☢☢☢ MRA head without and with IV contrast Usually Not Appropriate O MRA head without IV contrast Usually Not Appropriate O MRI head without and with IV contrast Usually Not Appropriate O MRI head without IV contrast Usually Not Appropriate O Radiography skull Usually Not Appropriate ☢ Variant 2: Child. Minor acute blunt head trauma. Intermediate risk for clinically important brain injury per PECARN criteria. Excluding suspected abusive head trauma. Initial imaging. Procedure Appropriateness Category Relative Radiation Level CT head without IV contrast May Be Appropriate ☢☢☢ Arteriography cerebral Usually Not Appropriate ☢☢☢☢ CT head with IV contrast Usually Not Appropriate ☢☢☢ CT head without and with IV contrast Usually Not Appropriate ☢☢☢☢ CTA head with IV contrast Usually Not Appropriate ☢☢☢☢ MRA head without and with IV contrast Usually Not Appropriate O MRA head without IV contrast Usually Not Appropriate O MRI head without and with IV contrast Usually Not Appropriate O MRI head without IV contrast Usually Not Appropriate O Radiography skull Usually Not Appropriate ☢ ACR Appropriateness Criteria® 1 Head Trauma-Child Variant 3: Child. Minor acute blunt head trauma. High risk for clinically important brain injury per PECARN criteria. -

Head Injury Pediatric After-Hours Version - Standard - 2015
Head Injury Pediatric After-Hours Version - Standard - 2015 DEFINITION Injuries to the head including scalp, skull and brain trauma INITIAL ASSESSMENT QUESTIONS 1. MECHANISM: "How did the injury happen?" For falls, ask: "What height did he fall from?" and "What surface did he fall against?" (Suspect child abuse if the history is inconsistent with the child's age or the type of injury.) 2. WHEN: "When did the injury happen?" (Minutes or hours ago) 3. NEUROLOGICAL SYMPTOMS: "Was there any loss of consciousness?" "Are there any other neurological symptoms?" 4. MENTAL STATUS: "Does your child know who he is, who you are, and where he is? What is he doing right now?" 5. LOCATION: "What part of the head was hit?" 6. SCALP APPEARANCE: "What does the scalp look like? Are there any lumps?" If so, ask: "Where are they? Is there any bleeding now?" If so, ask: "Is it difficult to stop?" 7. SIZE: For any cuts, bruises, or lumps, ask: "How large is it?" (Inches or centimeters) 8. PAIN: "Is there any pain?" If so, ask: "How bad is it?" 9. TETANUS: For any breaks in the skin, ask: "When was the last tetanus booster?" - Author's note: IAQ's are intended for training purposes and not meant to be required on every call. TRIAGE ASSESSMENT QUESTIONS Call EMS 911 Now [1] Major bleeding (actively dripping or spurting) AND [2] can't be stopped FIRST AID: apply direct pressure to the entire wound with a clean cloth CA: 50, 10 [1] Large blood loss AND [2] fainted or too weak to stand R/O: impending shock FIRST AID: have child lie down with feet elevated CA: 50, -

Firearm-Related Musculoskeletal Injuries in Children and Adolescents
Review Article Firearm-related Musculoskeletal Injuries in Children and Adolescents Abstract Cordelia W. Carter, MD Firearm injuries are a major cause of morbidity and mortality among Melinda S. Sharkey, MD children and adolescents in the United States and take financial and emotional tolls on the affected children, their families, and society as a Felicity Fishman, MD whole. Musculoskeletal injuries resulting from firearms are common and may involve bones, joints, and neurovascular structures and other soft tissues. Child-specific factors that must be considered in the setting of gunshot injuries include physeal arrest and lead toxicity. Understanding the ballistics associated with various types of weaponry is useful for guiding orthopaedic surgical treatment. Various strategies for preventing these injuries range from educational programs to the enactment of legislation focused on regulating guns and gun ownership. Several prominent medical societies whose members routinely care for children and adolescents with firearm- related injuries, including the American Academy of Pediatrics and the American Pediatric Surgical Association, have issued policy statements aimed at mitigating gun-related injuries and deaths in children. Healthcare providers for young patients with firearm-related musculoskeletal injuries must appreciate the full scope of this important public health issue. From the Department of Orthopaedics and Rehabilitation, Yale School of ecently, firearm-related injuries school campus or grounds, as Medicine, New Haven, CT. Dr. Carter in children and adolescents have documented by the press and con- or an immediate family member R serves as a board member, owner, been featured prominently in the firmed through further inquiries with officer, or committee member of the media. -

Trauma Service Area - B (BRAC)
Trauma Service Area - B (BRAC) Regional Pediatric Plan Trauma Service Area- B (BRAC) P.O. Box 53597 Lubbock, Texas 79453 806.791.2582 (office) BRAC serves the counties of Bailey, Borden, Castro, Cochran, Cottle, Crosby, Dawson, Dickens, Floyd, Gaines, Garza, Hale, Hockley, Kent, King, Lamb, Lubbock, Lynn, Motley, Scurry, Terry, Yoakum Revised 07/2016 1 Table of Contents Definition of Terms………………………………………………pg 3 Introduction.................................................................................... pg 4 Mission............................................................................................ pg 4 Vision............................................................................................... pg 5 Organization................................................................................... pg 5 Regional Plan.................................................................................. pg 5 Objectives........................................................................................ pg 5 Needs Analysis................................................................................ pg5 Goals............................................................................................... pg 6 Regulatory Agencies for Trauma Center Designation.............. pg 6 Pre-Hospital Triage....................................................................... pg 6 System Triage................................................................................ pg 7 Helicopter Activation................................................................... -

Trauma Quality Improvement Program (TQIP) Bibliography
Trauma Quality Improvement Program (TQIP) Bibliography The following bibliography includes citations found through PubMed searches, as well as those supplied by TQIP researchers in response to follow up surveys and e-mails. The list below includes publications, articles in press, and presentations in which TQIP is mentioned. This list is not exhaustive. If you have an TQIP-related publication that is not listed below, please provide the citation to [email protected]. Year Citation Subject/Keywords Badhiwala JH, Lebovic G, Balas M, da Costa L, Nathens AB, Fehlings MG, Wilson JR, Witiw CD. Variability in time to surgery 2021 for patients with acute thoracolumbar spinal cord injuries. Sci Rep. 2021 Jun 25;11(1):13312. doi: 10.1038/s41598-021-92310-z. SCI, Surgical Intervention PMID: 34172757. Owattanapanich N, Schellenberg M, Switzer E, Clark DH, Matsushima K, Inaba K. Association of Body Mass Index on Injuries 2021 and Outcomes After Ground-Level Falls. Am Surg. 2021 Jun 15:31348211024640. doi: 10.1177/00031348211024640. Epub Ground-level Fall, BMI ahead of print. PMID: 34130513. Stopenski S, Grigorian A, Inaba K, Lekawa M, Matsushima K, Schellenberg M, Kim D, de Virgilio C, Nahmias J. Prehospital 2021 Variables Alone Can Predict Mortality After Blunt Trauma: A Novel Scoring Tool. Am Surg. 2021 Jun 15:31348211024192. doi: Blunt Trauma, Triage 10.1177/00031348211024192. Epub ahead of print. PMID: 34128401. Anand T, Obaid O, Nelson A, Chehab M, Ditillo M, Hammad A, Douglas M, Bible L, Joseph B. Whole Blood Hemostatic 2021 Resuscitation in Pediatric Trauma: A Nationwide Propensity-Matched Analysis. J Trauma Acute Care Surg. -

A Trauma Assessment Pathway (TAP)
Assessment-Based Treatment for Traumatized Children: A Trauma Assessment Pathway (TAP) Chadwick Center for Children & Families Chadwick Center for Children and Families, Rady Children’s Hospital, San Diego The Chadwick Center for Children and Families is a Child Advocacy Center and department of Rady Children’s Hospital and Health Center in San Diego, CA. It is one of the largest centers of its kind and is staffed with more than 120 professionals and paraprofessionals in the field of medicine, social work, psychology, child development, nursing, and education technology. The Chadwick Center has made lasting differences in the lives of thousands of children and families since opening its doors in 1976. The staff is committed to family- centered care and a multidisciplinary approach to child abuse and family violence. The center’s mission is to promote the health and well-being of abused and traumatized children and their families. This is accomplished through excellence and leadership in evaluation, treatment, prevention, education, advocacy, and research. The center’s vision is to create a world where children and families are healthy and free from abuse and neglect. The National Child Traumatic Stress Network (NCTSN) Established by Congress in 2000, the National Child Traumatic Stress Network (NCTSN) is a unique collaboration of academic and community-based service centers whose mission is to raise the standard of care and increase access to services for traumatized children and their families across the United States. Combining knowledge of child development, expertise in the full range of child traumatic experiences, and attention to cultural perspectives, the NCTSN serves as a national resource for developing and disseminating evidence-based interventions, trauma-informed services, and public and professional education. -

Pediatric Polytrauma Management Robert M
ORIGINAL ARTICLE Pediatric Polytrauma Management Robert M. Kay, MD and David L. Skaggs, MD Abstract: Appropriate care of pediatric polytrauma patients requires GENERAL APPROACH TO the knowledge and expertise of a variety of subspecialists. Though POLYTRAUMA PATIENTS most of pediatric polytrauma patients survive, long-term sequelae The philosophy behind trauma centers resulted from the are common. The most common causes of long-term functional de- wartime experience showing that injured soldiers who were ficits after pediatric polytrauma involve injuries to the central ner- rapidly mobilized and transported for acute, emergency care vous and musculoskeletal systems. Orthopaedic care of polytrauma fared better than did those treated locally by less sophisticated patients is important to facilitate early mobilization and care of these teams and facilities. There is some evidence that care at children, as well as to minimize late impairment. pediatric trauma centers may result in lower mortality than at community hospitals,6 though the high costs of such centers Key Words: pediatric, polytrauma, orthopaedics and geographic realities have limited the number of such (J Pediatr Orthop 2006;26:268Y277) centers. Partly due to the limited number of pediatric trauma centers, adult trauma centers are often used to care for chil- dren who sustain polytrauma. Initial stabilization of the child is carried out by emer- rauma remains the leading cause of death in children over gency medical technicians in the field and is comparable to T1 year of age. This is true throughout the world, even in that for adults. The child is rapidly stabilized, fractures are societies with the most advanced medical systems, including often splinted, and the child is brought as quickly as possible state-of-the-art pediatric trauma centers. -

Penetrating Abdominal Injuries in Children: a Study of 33 Cases Samuel Wabada, Auwal M
8 Original article Penetrating abdominal injuries in children: a study of 33 cases Samuel Wabada, Auwal M. Abubakar, John Y. Chinda, Sani Adamu and Kefas J. Bwala Background Trauma is gradually becoming a major cause arrow injuries. Fourteen (42.4%) patients had abdominal of disability and it can be of any form, physical or organ evisceration; of them, nine were as a result of emotional. For the surgeon the physical form is of major gunshot injuries. Routine exploratory laparotomy was interest, especially its causes and incidence, which can be carried out in all 33 patients. Seven (21.2%) were operated influenced by environmental or social factors. on with simultaneous resuscitation in the immediate laparotomy group, and 26 (78.8%) underwent delayed Aim The aim of this work was to study the incidence, laparotomy. There was a negative laparotomy in four etiology, principles of management and outcome of (12.1%) patients, two of whom had only omental children with penetrating abdominal injuries. evisceration with no other accompanying visceral injuries, Materials and methods This was a 2-year prospective and two without evisceration. Three (9.1%) patients died study of 33 children aged 0–15 years with penetrating after developing enterocutaneous fistula, compartment abdominal injuries at the University of Maiduguri Teaching syndrome and sepsis. Hospital in northeast Nigeria. Information obtained Conclusion There were more cases of penetrating included the following: the patient’s biodata, mechanism of abdominal injuries among boys and children from the rural injury, time of presentation to the Accident and Emergency areas than in those from urban areas. Ann Pediatr Surg Department after the injury, haemodynamic status at 14:8–12 c 2018 Annals of Pediatric Surgery. -

Abusive Head Trauma: Evidence, Obfuscation, and Informed Management
LITERATURE REVIEW J Neurosurg Pediatr 24:481–488, 2019 Abusive head trauma: evidence, obfuscation, and informed management JNSPG 75th Anniversary Invited Review Article Ann-Christine Duhaime, MD,1 and Cindy W. Christian, MD2 1Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts; and 2Department of Pediatrics, Children’s Hospital of Philadelphia, The Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania Abusive head trauma remains the major cause of serious head injury in infants and young children. A great deal of research has been undertaken to inform the recognition, evaluation, differential diagnosis, management, and legal interventions when children present with findings suggestive of inflicted injury. This paper reviews the evolution of current practices and controversies, both with respect to medical management and to etiological determination of the variable constellations of signs, symptoms, and radiological findings that characterize young injured children presenting for neurosurgical care. https://thejns.org/doi/abs/10.3171/2019.7.PEDS18394 KEYWORDS child abuse; abusive head trauma; traumatic brain injury; subdural hematoma; nonaccidental trauma; shaken baby; infant ONACCIDENTAL trauma in infants and young chil- some infants are handled violently on multiple occasions dren remains a common problem associated with prior to presenting for medical care, while others are in- unique challenges and intervention opportunities jured in a single violent event.2 forN neurosurgeons, other clinicians, child advocates, and legal professionals. Research has led to substantial prog- Terminology and Mechanisms ress in understanding the epidemiology, clinical presenta- tion, evaluation, differential diagnosis, likely mechanisms, The terms “abusive head trauma,” “nonaccidental trau- pathophysiology, management, legal issues, and outcomes ma,” and “inflicted injury” are used interchangeably to of children with this constellation of injuries.