Assessing the Effect of Magnolia Macrophylla's Stigmatic Secretion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -
Trees and Shrubs for Bees Larger Trees, Mostly
These trees and shrubs are rated as good •, better!, and best ", roughly graded on the pollen and nectar forage they provide at a given time of year and as compared to other sources. What they actually Trees and Shrubs for Bees provide will vary depending on soil conditions, the weather in a given year, etc. These lists are not complete, though they include many of the best for our region. Other species, not listed here, are probably better than nothing. Invasive species are not listed. Native species, important Trees and shrubs are an important source of honeybee forage. In city for the habitat in general, are rated a little higher. and suburban locations around Philadelphia, tree planting helps to boost what's available for the bees, over and above what else might bloom in Before you plant, it's important to find out more about the specific tree yards and gardens. Carefully tended for a few years, trees will give back or shrub — the soil conditions it may require, the size it may grow to, for decades. Specific trees can provide early pollen, boost the nectar etc. References and Online Resources can give you more information. A flow, and offset the 'dearth' in late summer going into fall. more complete list of species and links to other resources are posted on the Philadelphia Beekeepers Guild website. Across the country, habitat losses are understood to be one of the most important factors damaging the health and well-being of honey bees. Larger trees, mostly Other insects, including America's native pollinators, are likewise MAPLE (ACER SPP.) affected. -

Foundation Arboretum Wespelaar Year Report 2019
Foundation Arboretum Wespelaar Year Report 2019 2019 started with an early spring: by mid-March the early magnolias were in full flower. Temperatures kept rising and the heat record was broken in July, making the summer of 2019 one to be recorded in history books. Luckily, small rain showers and increased irrigation capacities ensured that we lost very few plants. In March 2019, the construction of the Artois Pavilion started in the Artois Meadow, at the end of two important vistas. The design is inspired by the old pavilion along the canal in the Park of Wespelaar. In the new Arboretum de Marche, a total of 189 trees have been planted in the past two years. In collaboration with Natagora, five ponds were created and a 5ha plot was sown with wild seed to create a meadow of native grasses and flowers. The building permit for the visitors centre and technical facilities was obtained. 2019 was a very active year for both Arboretum Wespelaar and Arboretum de Marche, as is described in more detail in this Year Report. Arboretum Wespelaar – Year Report 2019 1 THE COLLECTIONS ‘Patty’ can be planted in the Arboretum in due time. The living collection of woody plants in the Arboretum currently (as of 30 January 2019) Once again, our winter was not worth contains 5,116 specimens representing 2,340 mentioning and by mid-March we could have different taxa (versus 16,376 specimens and opened the Arboretum for our visitors because 4,955 taxa on the whole of the estate). These the early magnolias were already in full flower! numbers include the 629 new accessions on On March 11th however, we had a serious the estate during 2019 of which 108 (or 17%) storm with accompanying damage and much are of documented wild origin. -

Plant-Pollinator Interactions in an Ecological and Evolutionary Context: the Promising
Plant-Pollinator Interactions in an Ecological and Evolutionary Context: The Promising Role of 3D-Printing Technology and Mathematical Modeling Eric Octavio Campos A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2017 Reading Committee: Thomas L. Daniel, Chair H.D. ‘Toby’ Bradshaw Janneke Hille Ris Lambers Program Authorized to Offer Degree: Biology ©Copyright 2017 Eric Octavio Campos University of Washington Abstract Plant-Pollinator Interactions in an Ecological and Evolutionary Context: The Promising Role of 3D-Printing Technology and Mathematical Modeling Eric Octavio Campos Co-Chairs of the Supervisory Committee: Professor H.D. ‘Toby’ Bradshaw Department of Biology Professor Thomas L. Daniel Department of Biology This dissertation concerns itself with the role of flower shape in affecting the foraging performance of pollinating animals. The pollinator used in this study is a model organism representing crepuscular hawkmoths in research involving the study of flight neuromuscular physiology and plant-pollinator interactions, Manduca sexta (hereafter Manduca). The broader goal of the work is to develop a new experimental framework for investigating the ecological and evolutionary consequences of plant-pollinator interactions. To that end, I have combined 3D-printing technology and mathematical modelling to construct artificial flowers, which can be manufactured with great precision and with objective, quantitatively describable shapes. First, I present a proof-of-concept study to demonstrate the feasibility of collecting foraging data from a real animal pollinator attempting to feed from 3D-printed artificial flowers. I show that Manduca’s foraging performance is extremely sensitive to variation in floral corolla curvature and nectary diameter. -

Magnolia Macrophylla: Bigleaf Magnolia1 Edward F
ENH-540 Magnolia macrophylla: Bigleaf Magnolia1 Edward F. Gilman and Dennis G. Watson2 Introduction green above with a fuzzy, silver/grey underside, creating a beautiful, two-toned effect with each passing breeze. From This North American native tree is deciduous in most areas May to July the showy, fragrant blossoms appear, each 8 to but semi-evergreen in the Deep South. Bigleaf Magnolia 12-inch-wide, ivory-colored bloom having a slight rose tint grows slowly to 30 to 40 feet and spreads 20 to 25 feet at its base. These blooms are followed by the production forming a rounded, broad canopy. The leaves of Bigleaf of 2.5 to 3-inch-long, hairy, red, egg-shaped fruits. Bigleaf Magnolia are truly large, 12 to 32 inches long and 7 to Magnolia trees must be 12 to 15-years-of-age before they 12 inches wide, when found in the wild and somewhat begin to bloom. smaller when grown in landscapes. These leaves are bright General Information Scientific name: Magnolia macrophylla Pronunciation: mag-NO-lee-uh mack-roe-FILL-uh Common name(s): Bigleaf Magnolia Family: Magnoliaceae USDA hardiness zones: 5B through 8B (Fig. 2) Origin: native to North America Figure 1. Young Magnolia macrophylla: Bigleaf Magnolia Credits: Ed Gilman Figure 2. Range 1. This document is ENH-540, one of a series of the Environmental Horticulture, UF/IFAS Extension. Original publication date November 1993. Revised December 2006. Reviewed February 2014. Visit the EDIS website at http://edis.ifas.ufl.edu. 2. Edward F. Gilman, professor, Environmental Horticulture Department; Dennis G. -

Diverse Nectar Robbers on Alpinia Roxburghii Sweet (Zingiberaceae)
Journal of Asia-Pacific Biodiversity 8 (2015) 238e241 HOSTED BY Contents lists available at ScienceDirect Journal of Asia-Pacific Biodiversity journal homepage: http://www.elsevier.com/locate/japb Short communication Diverse nectar robbers on Alpinia roxburghii Sweet (Zingiberaceae) Xiaobao Deng a, Wen Deng b, Alice Catherine Hughes c, Dharmalingam Mohandass a,* a Key Laboratory of Tropical Forest Ecology, Chinese Academy of Sciences, Menglun Town, Yunnan, PR China b Kunming Institute of Zoology, Chinese Academy of Sciences, Jiaochang Donglu, Kunming, Yunnan, PR China c Centre for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun Town, Yunnan, PR China article info abstract Article history: This study records for the first time three mammal species as nectar robbers on the ginger Alpinia Received 29 April 2015 roxburghii Sweet. We examined the behavior of nectar robbers and compared with earlier studies on a Received in revised form single plant species. We recorded seven species of nectar robbers: three squirrels, one bird, and three 29 July 2015 bees. Timing of robbing nectars were similar; however, robbing behavior differed among robbers. In Accepted 30 July 2015 particular, squirrels damaged the flower parts while robbing the nectar. Available online 18 August 2015 Copyright Ó 2015, National Science Museum of Korea (NSMK) and Korea National Arboretum (KNA). Production and hosting by Elsevier. This is an open access article under the CC BY-NC-ND license (http:// Keywords: animal behavior creativecommons.org/licenses/by-nc-nd/4.0/). ginger plant mammal-nectar robbers tropical seasonal rainforest Introduction studied in detail. Therefore, nectar robbers on ginger species could be a relevant topic to understand ecological consequences. -

Soil Characteristics of Natural Silver Linden (Tilia Tomentosa Moench) Populations
PEER-REVIEWED ARTICLE bioresources.com Soil Characteristics of Natural Silver Linden (Tilia tomentosa Moench) Populations Salih Parlak,* and Erdem Tetik Studies regarding the determination of the ecological characteristics of the natural distribution areas of the silver linden (Tilia tomentosa Moench) are limited. It is of great importance to select areas with similar natural cultivation characteristics in the plantations established for flower or timber production. Physiographical factors affecting these forests were explored to determine the physical and chemical characteristics of the soil. The soil samples were collected from three natural populations, and a total of 43 samples were examined in terms of aspect, elevation, declivity position, and slope. It was determined that the natural linden populations expanded between the altitudes of 0 m and 400 m and 88% of the populations were denser in aspects with shadow. It was found that 91% of the soil was in the class of “deep to very deep”, 61% showed an expansion in sandy clay loam soils, and 30% showed an expansion in sandy loam soils. Average soil pH ranged between 5.6 and 6.6. The soils were found to be salt-free and slightly limy. In terms of the organic carbon amount, the soils were classified as medium. Keywords: Tilia tomentosa; Silver linden; Natural expansion; Soil characteristics Contact information: Bursa Technical University, Faculty of Forestry, Department of Forest Engineering, 16310, Bursa, Turkey; *Corresponding author: [email protected] INTRODUCTION Turkey is comprised of three different plant regions, namely the Mediterranean, Europe-Siberia, and Irano-Turanian. Each one of these regions has its own endemic species and natural ecosystems (Tan 2010). -

Meristems West Tisbury, Massachusetts Vol
The Polly Hill Arboretum Meristems West Tisbury, Massachusetts Vol. 16, No. 2 Fall 2014 Ilex opaca ‘Villanova’: a yellow-fruited American holly selected and named by Polly Hill. Education Center Receives Matching Grant PHA continues to grow at a measured Situated at the heart of the Arboretum qualities of plants inspires our day-to-day pace. In 2006 the Arboretum outlined sev - campus between the Homestead (our work. Coupled with this is our desire eral capital projects to advance our mission administrative offices) and the Cowbarn, to share our enthusiasm and knowledge of of education, horticultural experimentation, the Education Center and Botany Lab plants with a larger group of children and plant conservation. We have been will provide a climate-controlled indoor and adults. The proposed building helps successful with a new greenhouse (2006), environment to extend our education us accomplish all these goals. the Cowbarn renovation (2007), the programming year-round and the space and Now the amazing news! PHA has Littlefield Maintenance Building (2009), equipment to advance our plant research. received a $500,000 gift from the Cedar and a refurbished Far Barn (2011). Its central location is visible from State Tree Foundation (the family foundation Today one significant project remains: Road, making its design and positioning of our founder, Dr. David Smith) to raise the proposed Education Center and Botany critical to maintaining the spirit of our matching funds for this new building. Lab. We have completed plans for this historical landscape. This generous grant supports our most building slated to replace the dilapidated PHA staff and board of directors are important education and scientific building outbuilding known as the Gym. -

Effect of Mutualistic and Antagonistic Bees on Floral Resources and Pollination of a Savanna Shrub
Flora 232 (2017) 30–38 Contents lists available at ScienceDirect Flora j ournal homepage: www.elsevier.com/locate/flora Effect of mutualistic and antagonistic bees on floral resources and ଝ pollination of a savanna shrub a a,b c c,∗ Marília Monteiro Quinalha , Anselmo Nogueira , Gisela Ferreira , Elza Guimarães a Graduation Program in Biological Sciences (Botany), Institute of Biosciences, UNESP – Univ Estadual Paulista, Botucatu, SP, Brazil b Centro de Ciências Naturais e Humanas, Universidade Federal do ABC, São Bernardo do Campo, SP, Brazil c Departament of Botany, Institute of Biosciences, UNESP – Univ Estadual Paulista, Botucatu, SP, Brazil a r t i c l e i n f o a b s t r a c t Article history: Since Darwin, cheaters have been described in plant-pollinator mutualisms. Bignoniaceae species have a Received 24 May 2016 wide interaction network with floral visitors, and most of those interactions are established with cheaters. Received in revised form 26 August 2016 Thus, our objective was to determine which role each floral visitor plays in a system composed by bees Accepted 30 August 2016 and a Bignoniaceae savanna species. So, here we described the bees’ behaviour and defined experi- Edited by S.D. Johnson mentally who are the mutualists and cheaters, we described the temporal sequence of interactions, Available online 6 September 2016 quantified pollen and nectar removal, and checked for the potential effect of robbery damages on pol- linator behaviour. Pollinators visited a small number of flowers, mainly in the early morning, while the Keywords: Bignoniaceae most frequent cheaters (robbers and thieves) visited the flowers throughout the day, increasing visi- Cheaters tation at midmorning, when pollinators had already visited the flowers. -
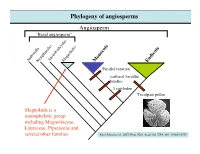
Phylogeny of Angiosperms Angiosperm “Basal Angiosperm”
Phylogeny of angiosperms Angiosperm “Basal angiosperm” AmborellaNymphaealesAustrobaileyalesMagnoliidss Monocots Eudicots Parallel venation scattered vascular bundles 1 cotyledon Tricolpate pollen Magnoliids is a monophyletic group including Magnoliaceae, Lauraceae, Piperaceae and several other families After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374 Magnoliaceae (Magnolia family) Textbook DVD KRR Magnolia X soulangeana; Magnoliaceae (Magnolia family) Textbook DVD WSJ Textbook DVD KRR Magnolia grandiflora; Magnolia macrophylla; Note leaf simple, entire, pinnate venation, numerous tepals, numerous stamens and carples. Textbook DVD KRR Magnolia sieboldii; Magnoliaceae (Magnolia family) Textbook DVD KMN Textbook DVD SMK-KRR Magnolia figo; Magnolia grandiflora; Note the elongated receptacle, Note the aggregate of follicles, and laminar stamens and red fleshy seed coat Magnoliaceae (Magnolia family) Photo: Yaowu Yuan Photo: Yaowu Yuan Liriodendron tulipifera; Note the elongated receptacle, and laminar stamens Magnoliaceae (Magnolia family) Note the lobed, T-shirt-like leaf, and pinnate venation Note the aggregate of samara Magnoliaceae (Magnolia family) Magnoliaceae - 2 genera/220 species. Trees or shrubs; Ethereal oils (aromatic terpenoids) - (remember the smell of bay leaves?); Leaves alternate, simple (Magnolia) or lobed (Liriodendron), entire; Flowers large and showy, actinomorphic, bisexual Tepals 6-numerous, stamens and carpels numerous, Spirally arranged on an elongated receptacle, Laminar stamens poorly differentiated into anther and filament. Fruit usually an aggregate of follicle (Magnolia) or samara (Liriodendron); follicle: 1-carpellate fruit that dehisces on the side samara: 1-carpellate winged, indehiscent fruit Phylogeny of Eudicots (or Tricolpates) Eudicots (or Tricolpates) “Basal eudicots” Asterids Buxales Rosids Caryophyllales RanunculalesProteales Ranunculales is a monophyletic group including Ranunculaceae, Berberidaceae, Papaveraceae, and 4 other families. After Jansen et al., 2007, Proc. -

Cheaters Must Prosper: Reconciling Theoretical and Empirical Perspectives on Cheating in Mutualism
Ecology Letters, (2015) 18: 1270–1284 doi: 10.1111/ele.12507 REVIEW AND SYNTHESIS Cheaters must prosper: reconciling theoretical and empirical perspectives on cheating in mutualism Abstract Emily I. Jones,1,2,3† Cheating is a focal concept in the study of mutualism, with the majority of researchers considering Michelle E. Afkhami,4 Erol Akßcay,5 cheating to be both prevalent and highly damaging. However, current definitions of cheating do Judith L. Bronstein,6 Redouan not reliably capture the evolutionary threat that has been a central motivation for the study of Bshary,7 Megan E. Frederickson,4 cheating. We describe the development of the cheating concept and distill a relative-fitness-based Katy D. Heath,8 Jason D. definition of cheating that encapsulates the evolutionary threat posed by cheating, i.e. that chea- Hoeksema,9 Joshua H. Ness,10 ters will spread and erode the benefits of mutualism. We then describe experiments required to 11 conclude that cheating is occurring and to quantify fitness conflict more generally. Next, we dis- M. Sabrina Pankey, Stephanie S. ‡ cuss how our definition and methods can generate comparability and integration of theory and Porter,12 Joel L. Sachs,12 Klara experiments, which are currently divided by their respective prioritisations of fitness consequences Scharnagl13 and Maren L. and traits. To evaluate the current empirical evidence for cheating, we review the literature on sev- Friesen13*,† eral of the best-studied mutualisms. We find that although there are numerous observations of low-quality partners, there is currently very little support from fitness data that any of these meet our criteria to be considered cheaters. -

Tilia Tomentosa
Woody Plants Database [http://woodyplants.cals.cornell.edu] Species: Tilia tomentosa (til'ee-ah toe-men-tose'ah) Silver Linden Cultivar Information A few cultivars are available for this superior species and it is likely that more will be developed in the future. Several cultivars available in Europe are not yet found in the US. * See specific cultivar notes on next page. Ornamental Characteristics Size: Tree > 30 feet Height: 60' - 80' Leaves: Deciduous Shape: pyramidal Ornamental Other: Environmental Characteristics Light: Full sun Hardy To Zone: 5a Soil Ph: Can tolerate acid to alkaline soil (pH 5.0 to 8.0) Environmental Other: tolerates drought and heat better than T. cordata CU Structural Soil™: Yes Insect Disease less susceptible to Japanese beetles than other lindens Bare Root Transplanting Difficult Other native to southeastern Europe, western Asia; transplant in spring; slower to recover from transplanting than 1 Woody Plants Database [http://woodyplants.cals.cornell.edu] other lindens Moisture Tolerance Occasionally saturated Consistently moist, Occasional periods of Prolonged periods of or very wet soil well-drained soil dry soil dry soil 1 2 3 4 5 6 7 8 9 10 11 12 2 Woody Plants Database [http://woodyplants.cals.cornell.edu] Cultivars for Tilia tomentosa Showing 1-7 of 7 items. Cultivar Name Notes Sterling Silver 'Sterling Silver' - vigorous grower; resistant to Japanese beetles Green Mountain 'Green Mountain' - symmetrical; dense canopy Sashazam 'Satin Shadow' (a.k.a. 'Sashazam') - symmetrical, reportedly resistant to Japanese