Exploring Molecular Mechanisms of Itch and Touch in the Mammalian Somatosensory System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 TRP About Online
a TR P to Spain International Workshop on Transient Receptor Potential Channels 12th – 14th September 2012 Valencia, Spain www.trp2012.com SCHEDULE and ABSTRACTS BOOK September 2012 Dear participants, Travelling to faraway places in search of spiritual or cultural enlightenment is a millennium old human activity. In their travels, pilgrims brought with them news, foods, music and traditions from distant lands. This friendly exchange led to the cultural enrichment of visitors and the economic flourishing of places, now iconic, such as Rome, Santiago, Jerusalem, Mecca, Varanasi or Angkor Thom. The dissemination of science and technology also benefited greatly from these travels to remote locations. The new pilgrims of the Transient Receptor Potential (TRP) community are also very fond of travelling. In the past years they have gathered at various locations around the globe: Breckenridge (USA), Eilat (Israel), Stockholm (Sweden) and Leuven (Belgium) come to mind. These meetings, each different and exciting, have been very important for the dissemination of TRP research. We are happy to welcome you in Valencia (Spain) for TRP2012. The response to our call has been extraordinary, surpassing all our expectations. The speakers, the modern bards, readily attended our request to communicate their new results. At last count we were already more than 170 participants, many of them students, and most presenting their recent work in the form of posters or short oral presentations. At least 25 countries are sending TRP ambassadors to Valencia, making this a truly international meeting. We like to thank the staff of the Cátedra Santiago Grisolía, Fundación Ciudad de las Artes y las Ciencias for their dedication and excellence in handling the administrative details of the workshop. -

The Mineralocorticoid Receptor Leads to Increased Expression of EGFR
www.nature.com/scientificreports OPEN The mineralocorticoid receptor leads to increased expression of EGFR and T‑type calcium channels that support HL‑1 cell hypertrophy Katharina Stroedecke1,2, Sandra Meinel1,2, Fritz Markwardt1, Udo Kloeckner1, Nicole Straetz1, Katja Quarch1, Barbara Schreier1, Michael Kopf1, Michael Gekle1 & Claudia Grossmann1* The EGF receptor (EGFR) has been extensively studied in tumor biology and recently a role in cardiovascular pathophysiology was suggested. The mineralocorticoid receptor (MR) is an important efector of the renin–angiotensin–aldosterone‑system and elicits pathophysiological efects in the cardiovascular system; however, the underlying molecular mechanisms are unclear. Our aim was to investigate the importance of EGFR for MR‑mediated cardiovascular pathophysiology because MR is known to induce EGFR expression. We identifed a SNP within the EGFR promoter that modulates MR‑induced EGFR expression. In RNA‑sequencing and qPCR experiments in heart tissue of EGFR KO and WT mice, changes in EGFR abundance led to diferential expression of cardiac ion channels, especially of the T‑type calcium channel CACNA1H. Accordingly, CACNA1H expression was increased in WT mice after in vivo MR activation by aldosterone but not in respective EGFR KO mice. Aldosterone‑ and EGF‑responsiveness of CACNA1H expression was confrmed in HL‑1 cells by Western blot and by measuring peak current density of T‑type calcium channels. Aldosterone‑induced CACNA1H protein expression could be abrogated by the EGFR inhibitor AG1478. Furthermore, inhibition of T‑type calcium channels with mibefradil or ML218 reduced diameter, volume and BNP levels in HL‑1 cells. In conclusion the MR regulates EGFR and CACNA1H expression, which has an efect on HL‑1 cell diameter, and the extent of this regulation seems to depend on the SNP‑216 (G/T) genotype. -

Advancing Basic Pain Research
The Rita Allen Foundation AWARD IN PAIN SCHOLARS: ADVANCING BASIC PAIN RESEARCH AWARD IN PAIN SCHOLARS: ADVANCING BASIC PAIN RESEARCH 1 COVER: Tuan Trang, a 2014 Rita Allen Foundation Scholar, has investigated mechanisms of opioid tolerance and withdrawal. Trang and his research group have found that immune cells in the central nervous system, known as microglia, play a key role in the development of morphine tolerance in an animal model. This image, a compilation of spinal microglia forming a cross section of the lumbar spinal cord, appeared on the cover of the October 18, 2017, issue of The Journal of Neuroscience in conjunction with the research article “Site-Specific Regulation of P2X7 Receptor Function in Microglia Gates Morphine Analgesic Tolerance.” (Image by Heather Leduc-Pessah, Trang Laboratory) A NETWORK OF HOPE Created in 2009 to expand the reach of the Rita Allen Foundation Scholars program, the Award in Pain has now supported 29 pioneering early-career Pain Scholars. Each year, as we welcome our newest class of Scholars, we reflect on the accomplishments of this growing community of researchers and the profound questions that drive them forward to new discoveries. These scientists are leading efforts to understand the complex neurobiological mechanisms that underlie pain—including mapping the neural circuits of chronic pain, defining the Elizabeth G. Christopherson roles of immune signals, and examining the connections President and between pain and itch. Their findings point to approaches for Chief Executive Officer combating opioid tolerance and withdrawal, interventions Rita Allen Foundation to interrupt the transition from acute to chronic pain after injury, and targets for completely novel pain therapies with the potential to improve safety and specificity. -

Potassium Channels in Epilepsy
Downloaded from http://perspectivesinmedicine.cshlp.org/ on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press Potassium Channels in Epilepsy Ru¨diger Ko¨hling and Jakob Wolfart Oscar Langendorff Institute of Physiology, University of Rostock, Rostock 18057, Germany Correspondence: [email protected] This review attempts to give a concise and up-to-date overview on the role of potassium channels in epilepsies. Their role can be defined from a genetic perspective, focusing on variants and de novo mutations identified in genetic studies or animal models with targeted, specific mutations in genes coding for a member of the large potassium channel family. In these genetic studies, a demonstrated functional link to hyperexcitability often remains elusive. However, their role can also be defined from a functional perspective, based on dy- namic, aggravating, or adaptive transcriptional and posttranslational alterations. In these cases, it often remains elusive whether the alteration is causal or merely incidental. With 80 potassium channel types, of which 10% are known to be associated with epilepsies (in humans) or a seizure phenotype (in animals), if genetically mutated, a comprehensive review is a challenging endeavor. This goal may seem all the more ambitious once the data on posttranslational alterations, found both in human tissue from epilepsy patients and in chronic or acute animal models, are included. We therefore summarize the literature, and expand only on key findings, particularly regarding functional alterations found in patient brain tissue and chronic animal models. INTRODUCTION TO POTASSIUM evolutionary appearance of voltage-gated so- CHANNELS dium (Nav)andcalcium (Cav)channels, Kchan- nels are further diversified in relation to their otassium (K) channels are related to epilepsy newer function, namely, keeping neuronal exci- Psyndromes on many different levels, ranging tation within limits (Anderson and Greenberg from direct control of neuronal excitability and 2001; Hille 2001). -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Transcriptomic Analysis of Native Versus Cultured Human and Mouse Dorsal Root Ganglia Focused on Pharmacological Targets Short
bioRxiv preprint doi: https://doi.org/10.1101/766865; this version posted September 12, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Transcriptomic analysis of native versus cultured human and mouse dorsal root ganglia focused on pharmacological targets Short title: Comparative transcriptomics of acutely dissected versus cultured DRGs Andi Wangzhou1, Lisa A. McIlvried2, Candler Paige1, Paulino Barragan-Iglesias1, Carolyn A. Guzman1, Gregory Dussor1, Pradipta R. Ray1,#, Robert W. Gereau IV2, # and Theodore J. Price1, # 1The University of Texas at Dallas, School of Behavioral and Brain Sciences and Center for Advanced Pain Studies, 800 W Campbell Rd. Richardson, TX, 75080, USA 2Washington University Pain Center and Department of Anesthesiology, Washington University School of Medicine # corresponding authors [email protected], [email protected] and [email protected] Funding: NIH grants T32DA007261 (LM); NS065926 and NS102161 (TJP); NS106953 and NS042595 (RWG). The authors declare no conflicts of interest Author Contributions Conceived of the Project: PRR, RWG IV and TJP Performed Experiments: AW, LAM, CP, PB-I Supervised Experiments: GD, RWG IV, TJP Analyzed Data: AW, LAM, CP, CAG, PRR Supervised Bioinformatics Analysis: PRR Drew Figures: AW, PRR Wrote and Edited Manuscript: AW, LAM, CP, GD, PRR, RWG IV, TJP All authors approved the final version of the manuscript. 1 bioRxiv preprint doi: https://doi.org/10.1101/766865; this version posted September 12, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

The Chondrocyte Channelome: a Novel Ion Channel Candidate in the Pathogenesis of Pectus Deformities
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 2017 The Chondrocyte Channelome: A Novel Ion Channel Candidate in the Pathogenesis of Pectus Deformities Anthony J. Asmar Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biology Commons, Molecular Biology Commons, and the Physiology Commons Recommended Citation Asmar, Anthony J.. "The Chondrocyte Channelome: A Novel Ion Channel Candidate in the Pathogenesis of Pectus Deformities" (2017). Doctor of Philosophy (PhD), Dissertation, Biological Sciences, Old Dominion University, DOI: 10.25777/pyha-7838 https://digitalcommons.odu.edu/biology_etds/19 This Dissertation is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. THE CHONDROCYTE CHANNELOME: A NOVEL ION CHANNEL CANDIDATE IN THE PATHOGENESIS OF PECTUS DEFORMITIES by Anthony J. Asmar B.S. Biology May 2010, Virginia Polytechnic Institute M.S. Biology May 2013, Old Dominion University A Dissertation Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY BIOMEDICAL SCIENCES OLD DOMINION UNIVERSITY August 2017 Approved by: Christopher Osgood (Co-Director) Michael Stacey (Co-Director) Lesley Greene (Member) Andrei Pakhomov (Member) Jing He (Member) ABSTRACT THE CHONDROCYTE CHANNELOME: A NOVEL ION CHANNEL CANDIDATE IN THE PATHOGENESIS OF PECTUS DEFORMITIES Anthony J. Asmar Old Dominion University, 2017 Co-Directors: Dr. Christopher Osgood Dr. Michael Stacey Costal cartilage is a type of rod-like hyaline cartilage connecting the ribs to the sternum. -
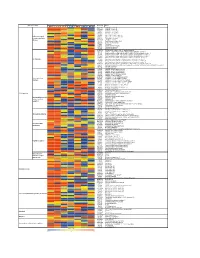
Supporting Figure 2
Normalized value Functional class Genbank Name Naive SC 1h SC 6h SC 24h WM 1h WM 6h WM 24h AB016161 GABA-B receptor 1d AF109405 GABA-B receptor 2a M35077 Dopamine-1A receptor S46131 Dopamine-1A receptor M84009 Dopamine receptor D4 U13368 Adrenergic receptor, alpha 1a G Protein-coupled M60654 Adrenergic receptor, alpha 1d receptors and their M64236 Tachykinin 1 receptor effectors AI229237 Opioid receptor-like Y11433 Pyrimidinergic receptor P2Y4 M64299 Adenosine A1 receptor E00001 Pro-insulin M29014 Insulin receptor precursor U35315 Serotonin receptor 2C S62043 Serotonin receptor 6 AF000368 Scn9a sodium channel, type IX, alpha polypeptide M27158 Kcna5 K+ voltage-gated channel, shaker-related subfamily, member 5 X17621 Kcna6 potassium voltage-gated channel, shaker-related, subfamily, member 6 X16476 Kcnb1 potassium voltage gated channel, Shab-related subfamily, member 1 M77482 Kcnb2 potassium voltage gated channel, Shab-related subfamily, member 2 S64320 Kcnd2 potassium voltage gated channel, Shal-related family, member 2 Ion channels X87635 Kcnj4 potassium inwardly-rectifying channel, subfamily J, member 4 D86039 Kcnj11 potassium inwardly-rectifying channel, subfamily J, member 11 X83581 Kcnj16 potassium inwardly-rectifying channel, subfamily J, member 16 AF073891 Kcnh5 potassium voltage-gated channel, subfamily H (eag-related), member 5 U69882 Kcnn2 potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 Z36944 Chloride channel 4-2 Z56277 Chloride channel 5 L08493 GABA-A receptor alpha-4 subunit X51992 -

Phenotypic Spectrum and Long-Term Outcome in Children with Genetic Causes of Early-Onset Epileptic Encephalopathy
Phenotypic Spectrum and Long-term Outcome in Children With Genetic Causes of Early-onset Epileptic Encephalopathy Chunhui Hu Department of Neurology, Children’s Hospital of Fudan University Deying Liu Wuhan Children’s hospital, Tongji Medical college, Huazhong University of Science & Technology Tian Luo Department of Neurology, Children’s Hospital of Fudan University Yi Wang ( [email protected] ) Department of Neurology, Children’s Hospital of Fudan University Zhisheng Liu Wuhan Children’s hospital, Tongji Medical college, Huazhong University of Science & Technology Research Article Keywords: Phenotypic spectrum, Long-term outcome, Genetic, EOEE, Therapy Posted Date: March 11th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-257334/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/23 Abstract Background To explore the clinical phenotype and long-term outcome in children with genetic causes of early-onset epileptic encephalopathies. Methods The clinical data of 118 children between 2010 and 2020 was obtained and analyzed. The whole exome sequencing and copy number variation studies in family were used to nd pathogenic mutations. The conrmed mutations were veried by Sanger sequencing. Results Among 118 patients, 39 patients were diagnosed with DS, 18 were WS, 3 were OS, 3 were EME, 2 were MMFSI, 1 was GLUT1 deciency syndrome, 1 was Pyridoxine dependent epilepsy and 51 were non-symptomatic EOEEs. The initial EEG showed frequent multiple and multifocal sharp waves, spike waves, sharp slow waves or spike slow waves. In the later period, some transformed into infrequent discharging or normal EEG. 112 patients (112/118, 94.9%) showed normal brain MRI, and the remaining 6 had widened extracerebral space. -

An Advance About the Genetic Causes of Epilepsy
E3S Web of Conferences 271, 03068 (2021) https://doi.org/10.1051/e3sconf/202127103068 ICEPE 2021 An advance about the genetic causes of epilepsy Yu Sun1, a, *, †, Licheng Lu2, b, *, †, Lanxin Li3, c, *, †, Jingbo Wang4, d, *, † 1The School of Molecular and Cellular Biology, University of Illinois at Urbana-Champaign, Urbana, IL 61801-3633, US 2High School Affiliated to Shanghai Jiao Tong University, Shanghai, 200441, China 3Applied Biology program, University of British Columbia, Vancouver, V6r3b1, Canada 4School of Chemical Machinery and Safety, Dalian University of Technology, Dalian, 116023, China †These authors contributed equally. Abstract: Human hereditary epilepsy has been found related to ion channel mutations in voltage-gated channels (Na+, K+, Ca2+, Cl-), ligand gated channels (GABA receptors), and G-protein coupled receptors, such as Mass1. In addition, some transmembrane proteins or receptor genes, including PRRT2 and nAChR, and glucose transporter genes, such as GLUT1 and SLC2A1, are also about the onset of epilepsy. The discovery of these genetic defects has contributed greatly to our understanding of the pathology of epilepsy. This review focuses on introducing and summarizing epilepsy-associated genes and related findings in recent decades, pointing out related mutant genes that need to be further studied in the future. 1 Introduction Epilepsy is a neurological disorder characterized by 2 Malfunction of Ion channel epileptic seizures caused by abnormal brain activity. 1 in Functional variation in voltage or ligand-gated ion 100 (50 million people) people are affected by symptoms channel mutations is a major cause of idiopathic epilepsy, of this disorder worldwide, with men, young children, and especially in rare genetic forms. -

Microarray Analysis of Colorectal Cancer Stromal Tissue Reveals Upregulation of Two Oncogenic Mirna Clusters
Published OnlineFirst March 27, 2012; DOI: 10.1158/1078-0432.CCR-11-1078 Clinical Cancer Human Cancer Biology Research Microarray Analysis of Colorectal Cancer Stromal Tissue Reveals Upregulation of Two Oncogenic miRNA Clusters Naohiro Nishida1,4, Makoto Nagahara2, Tetsuya Sato3, Koshi Mimori1, Tomoya Sudo1, Fumiaki Tanaka1, Kohei Shibata1, Hideshi Ishii1,4, Kenichi Sugihara2, Yuichiro Doki4, and Masaki Mori1,4 Abstract Purpose: Cancer stroma plays an important role in the progression of cancer. Although alterations in miRNA expression have been explored in various kinds of cancers, the expression of miRNAs in cancer stroma has not been explored in detail. Experimental Design: Using a laser microdissection technique, we collected RNA samples specific for epithelium or stroma from 13 colorectal cancer tissues and four normal tissues, and miRNA microarray and gene expression microarray were carried out. The expression status of miRNAs was confirmed by reverse transcriptase PCR. Furthermore, we investigated whether miRNA expression status in stromal tissue could influence the clinicopathologic factors. Results: Oncogenic miRNAs, including two miRNA clusters, miR-17-92a and miR-106b-25 cluster, were upregulated in cancer stromal tissues compared with normal stroma. Gene expression profiles from cDNA microarray analyses of the same stromal tissue samples revealed that putative targets of these miRNA clusters, predicted by Target Scan, such as TGFBR2, SMAD2, and BMP family genes, were significantly downregulated in cancer stromal tissue. Downregulated putative targets were also found to be involved in cytokine interaction and cellular adhesion. Importantly, expression of miR-25 and miR-92a in stromal tissues was associated with a variety of clinicopathologic factors. Conclusions: Oncogenic miRNAs were highly expressed in cancer stroma. -

Transcriptomic Profiling of Ca Transport Systems During
cells Article Transcriptomic Profiling of Ca2+ Transport Systems during the Formation of the Cerebral Cortex in Mice Alexandre Bouron Genetics and Chemogenomics Lab, Université Grenoble Alpes, CNRS, CEA, INSERM, Bâtiment C3, 17 rue des Martyrs, 38054 Grenoble, France; [email protected] Received: 29 June 2020; Accepted: 24 July 2020; Published: 29 July 2020 Abstract: Cytosolic calcium (Ca2+) transients control key neural processes, including neurogenesis, migration, the polarization and growth of neurons, and the establishment and maintenance of synaptic connections. They are thus involved in the development and formation of the neural system. In this study, a publicly available whole transcriptome sequencing (RNA-Seq) dataset was used to examine the expression of genes coding for putative plasma membrane and organellar Ca2+-transporting proteins (channels, pumps, exchangers, and transporters) during the formation of the cerebral cortex in mice. Four ages were considered: embryonic days 11 (E11), 13 (E13), and 17 (E17), and post-natal day 1 (PN1). This transcriptomic profiling was also combined with live-cell Ca2+ imaging recordings to assess the presence of functional Ca2+ transport systems in E13 neurons. The most important Ca2+ routes of the cortical wall at the onset of corticogenesis (E11–E13) were TACAN, GluK5, nAChR β2, Cav3.1, Orai3, transient receptor potential cation channel subfamily M member 7 (TRPM7) non-mitochondrial Na+/Ca2+ exchanger 2 (NCX2), and the connexins CX43/CX45/CX37. Hence, transient receptor potential cation channel mucolipin subfamily member 1 (TRPML1), transmembrane protein 165 (TMEM165), and Ca2+ “leak” channels are prominent intracellular Ca2+ pathways. The Ca2+ pumps sarco/endoplasmic reticulum Ca2+ ATPase 2 (SERCA2) and plasma membrane Ca2+ ATPase 1 (PMCA1) control the resting basal Ca2+ levels.