I General Introducti On
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Control of Growth by Picolinic Acid
Proc. Nati. Acad. Sci. USA Vol. 74, No. 7, pp. 2889-2893, July 1977 Cell Biology Control of growth by picolinic acid: Differential response of normal and transformed cells (cell synchrony/chelating agents/NAD+/pyridine derivatives/viral transformation) J. A. FERNANDEZ-POL*t, VINCENT H. BONO, JR.J, AND GEORGE S. JOHNSON* * Laboratory of Molecular Biology and * Laboratory of Medicinal Chemistry and Biology, National Cancer Institute, Bethesda, Maryland 20014 Communicated by Nathan 0. Kaplan, April 14, 1977 ABSTRACT Picolinic acid reversibly inhibits the growth under 95% air/5% CO2, humidified atmosphere, at 37°. The of cultured cells. Fourteen other pyridine derivatives were in- cell lines used are listed in Table 1. Unless otherwise indicated, effective or toxic. Untransformed normal rat kidney (NRK) cells cells were planted at 1.5 X 105 cells per dish. Twenty-four hours are reversibly arrested in the G1 stage of the growth cycle as later, media were replaced by new media with or shown by cell counts, mitotic index, [3H]thymidine incorpora- (treated) tion, and flow microfluorometry. Flow microfluorometry was without (control) picolinic acid. All cells lines were treated with used to monitor the effects of picolinic acid on numerous other picolinic acid at 1.5,2,2.5,3, and 4 mM and analyzed for 4 days cell lines. Normal cells are blocked in GI, whereas transformed with media change every other day. cells show responses that are dependent upon the transforming Cells were analyzed for DNA content by flow microfluo- virus and independent of species or origin of the cell line. Kir- rometry (FMF) after trypsinization and suspension in pro- sten sarcoma virus-transformed cells are blocked in GI. -

Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis ISBN 978 92 4 154880 9
Companion handbookCompanion tofor the the WHO programmatic guidelines management of drug-resistant tuberculosis Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis ISBN 978 92 4 154880 9 Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis This book is a companion handbook to existing WHO policy guidance on the management of multidrug-resistant tuberculosis, including the WHO guidelines for the programmatic management of drug-resistant tuberculosis, WHO interim policy guidance on the use of bedaquiline in the treatment of multidrug-resistant tuberculosis, and the WHO interim policy guidance on the use of delamanid in the treatment of multidrug-resistant tuberculosis which were developed in compliance with the process for evidence gathering, assessment and formulation of recommendations, as outlined in the WHO Handbook for Guideline Development (version March 2010; available at http://apps.who.int/iris/ bitstream/10665/75146/1/9789241548441_eng.pdf ). WHO Library Cataloguing-in-Publication Data Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. 1.Antitubercular agents – administration and dosage. 2.Tuberculosis, Multidrug-Resistant – drug therapy. 3.Treatment outcome. 4.Guideline. I.World Health Organization. ISBN 978 92 4 154880 9 (NLM classification: WF 360) © World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index. -

Picolinic and Isonicotinic Acids: a Fourier Transform Microwave Spectroscopy Study
Subscriber access provided by UNIV OF CALIFORNIA SAN DIEGO LIBRARIES Article Picolinic and Isonicotinic Acids: a Fourier Transform Microwave Spectroscopy Study Isabel Peña, Marcelino Varela, Vanina Franco, Juan Carlos Lopez, Carlos Cabezas, and Jose Luis Alonso J. Phys. Chem. A, Just Accepted Manuscript • DOI: 10.1021/jp509823v • Publication Date (Web): 10 Nov 2014 Downloaded from http://pubs.acs.org on November 17, 2014 Just Accepted “Just Accepted” manuscripts have been peer-reviewed and accepted for publication. They are posted online prior to technical editing, formatting for publication and author proofing. The American Chemical Society provides “Just Accepted” as a free service to the research community to expedite the dissemination of scientific material as soon as possible after acceptance. “Just Accepted” manuscripts appear in full in PDF format accompanied by an HTML abstract. “Just Accepted” manuscripts have been fully peer reviewed, but should not be considered the official version of record. They are accessible to all readers and citable by the Digital Object Identifier (DOI®). “Just Accepted” is an optional service offered to authors. Therefore, the “Just Accepted” Web site may not include all articles that will be published in the journal. After a manuscript is technically edited and formatted, it will be removed from the “Just Accepted” Web site and published as an ASAP article. Note that technical editing may introduce minor changes to the manuscript text and/or graphics which could affect content, and all legal disclaimers and ethical guidelines that apply to the journal pertain. ACS cannot be held responsible for errors or consequences arising from the use of information contained in these “Just Accepted” manuscripts. -

Microbial Transformation of Isonicotinic Acid Hydrazide and Isonicotinic Acid by Sarcina Sp*
J. Biosci., Vol. 1, Number 2, June 1979, pp. 223–234. © Printed in India. Microbial transformation of isonicotinic acid hydrazide and isonicotinic acid by Sarcina sp* R. C. GUPTA and O. P. SHUKLA Division of Biochemistry, Central Drug Research Institute, Lucknow 226 001 † Present address : Regional Research Laboratory, Jorhat, Assam. MS received 20 November 1978; revised 22 February 1979 Abstract. Metabolism of isonicotinic acid and isoniazid by Sarcina sp. led to the formation of two metabolites which were characterised as 2-hydroxyisonicotinic acid and citrazinic acid. The blue pigment formed during fermentation was shown to be derived from the auto-oxidation of citrazinic acid. 2-Oxo-glutarate accumulated as the major keto acid when isonicotinic acid or isonicotinic acid hydrazide meta- bolism was inhibited by 1 mM sodium arsenite. Isonicotinic acid, 2-hydroxy- isonicotinic acid and 2-oxo-glutarate were oxidised by isonicotinic acid hydrazide or isonicotinic acid-grown cells; citrazinic acid was, however, not oxidised. Isoniazid hydrazine hydrolase, isonicotinic acid and 2-hydroxyisonicotinic acid hydroxylases were detected in the cell-free extract of Sarcina sp. grown on isonicotinic acid hydrazide or isonicotinic acid. Keywords. Microbial transformation; isoniazid; isonicotinic acid; Sarcina. Introduction Metabolism of isonicotinic acid (INA) and N-methylisonicotinic acid has been investigated by Ensign and Rittenberg (1965), Orpin et al. (1972) and Wright and Cain (1972). Fishbain et al. (1972) reported the hydrolysis of isonicotinic acid hydrazide (INH) to INA and hydrazine by Mycobacterium smegmatis, but INA was not metabolised further. Metabolism of INH by M. tuberculosis has been reported to yield isonicotinic acid, pyridine-4-aldehyde and pyridine-4- methanol (Krishna Murti, 1974). -

Production of Isonicotinic Acid Using Agar Entrapped Whole Cells of Nocardia Globerula NHB-2 Bhalla T. C., Mehta P.K., Sharma N
XVIIth International Conference on Bioencapsulation, Groningen, Netherlands ; September 24-26, 2009 XVIIth International Conference on Bioencapsulation, Groningen, Netherlands ; September 24-26, 2009 Production of isonicotinic acid using agar entrapped whole cells of Nocardia isobutyronitrile in the growth medium containing 1% glucose, 0.5 % peptone, 0.3% beef extract globerula NHB-2 and 0.1% yeast extract , pH 7.5(Sharma N.N., 2009). The organism was cultured at 30°C in an incubator shaker at 160 rpm. The cells were sedimented at 10000 ‘g’ and suspended in 0.1M sodium Bhalla T. C., Mehta P.K., Sharma N.N. and Bhatia S.K. phosphate buffer, pH 7.5 and were termed as resting whole cells. The nitrilase activity of the Department of Biotechnology, Himachal Pradesh University, Summer Hill, resting cells was assayed as reported earlier using 4-cyanopyridine as substrate (Sharma N.N. et al. Shimla-171005, Himachal Pradesh, INDIA [email protected] 2006). One unit of nitrilase activity was defined as that amount of enzyme (whole cells) which catalyzed the conversion of one µ mole of 4-cyanopyrine to isonicotinic acid per min under the INTRODUCTION assay conditions. Isonicotinic acid or pyridine-4-carboxylic acid is an important pyridine derivative which is mainly The whole cells of N. globerula NHB-2 were immobilized in agar (1%, w/v) and small beads (4 used for the synthsis of isoniazid (an antituberculastic drug), inabenfide (a plant growth regulator), mm x 2.5 mm) were prepared following the methods described previously (Raj J. et al. 2007). The terefenadine (an antihistamine) and nialamide (an antidepressant) (Yadav G.D. -

M/S. Veer Chemie & Aromatics (P) Ltd. LIST of CONTENTS S.NO
M/s. Veer Chemie & Aromatics (P) Ltd. LIST OF CONTENTS S.NO DESCRIPTION Enclosures PAGE NO & Annexures Enclosures 1 Copy of EC Enclosure - 1 1-4 2 Copy of CFO Enclosure - 2 1-6 3 TSDF Membership Enclosure - 3 1-4 4 Plant Lay out Enclosure - 4 1 Annexures 1 List Of Products Annexure - I 2 2 Manufacturing Process Annexure - II 3-46 3 Water Consumption Details Annexure - III 47 4 Waste water Details Annexure - IV 48 5 Solid & Hazardous waste details Annexure - V 49 6 Stack Emission Details Annexure - VI 50-51 7 Process Emission Details Annexure -VII 52 8 List of Raw materials Annexure - VIII 53-57 9 Solvents Details Annexure - IX 58-59 Prepared By Rightsource Industrial Solutions Pvt. Ltd., Page 1 Enclosure - 1 Enclosure - 2 Enclosure - 3 Enclosure - 4 6 f'9L,i'$ 5 /*:t.7' \3 \ <} FA *Y S\ (.rlQ (J==E L6 ffif{i o3=5 <*d.e c5g sfi 6 E.; baB UJ= -J- + QXto ur+d+ LU nnZ= oo=OOz CNH SUNOSH9I]N zo 3 d. = o (9I U z ONfl SUNOSH9I]N M/s. Veer Chemie & Aromatics (P) Ltd. Annexure - I LIST OF EXISTING PRODUCTS S. No Product Name Quantity In Quantity In Kg/Month Kg/Day 1 Niacinamide 25000.00 833.33 2 Niacin & its derivatives 25000.00 833.33 3 Isoniazid 6000.00 200.00 56000.00 1866.67 LIST OF PROPOSED PRODUCTS S. No Product Name Quantity In Quantity In MT/Month MT/Day Group-A 1 Isonicotinic acid 325.00 10.83 2 Nicotinic acid 325.00 10.83 3 Niacinamide 325.00 10.83 Total (Sum of any one or all products 325.00 10.83 Group-B 1 Benzyl Nicotinate 50.00 1.67 2 Ethyl Isonicotinate 50.00 1.67 3 Ethyl Nicotinate 50.00 1.67 4 Hexyl Nicotinate 50.00 1.67 5 Methyl Isonicotinic acid 50.00 1.67 6 Methyl Nicotinate 50.00 1.67 7 Methyl-6-Methyl Nicotinate 50.00 1.67 8 Myristyl nicotinate 50.00 1.67 Total (Sum of any one or all products 50.00 1.67 Total Sum of (Group-A + Group-B) 375.00 12.50 Prepared By Rightsource Industrial Solutions Pvt. -
(12) United States Patent (10) Patent No.: US 6,734,309 B1 Ray Et Al
USOO6734309B1 (12) United States Patent (10) Patent No.: US 6,734,309 B1 Ray et al. (45) Date of Patent: May 11, 2004 (54) PROCESS FOR THE SYNTHESIS OF FOREIGN PATENT DOCUMENTS SONICOTINIC ACID HYDRAZIDE DE 1116 667 11/1961 (75) Inventors: Subhash Chandra Ray, Dhanbad (IN); Lakshmi Narayan Nandi, Dhanbad OTHER PUBLICATIONS (IN); Baldev Singh, Dhanbad (IN); Hiralal Prasad, Dhanbad (IN); Sumant Maharaj, Dhanbad (IN); Prodyot Database CrossFire Beilstein Online Beilstein Institut Zur Kumar Sarkar, Dhanbad (IN); Forderung der Chemischen Wissenschaften, Frankfurt am Pashupati Dutta, Dhanbad (IN); Main, DE; Database accession No. Reaction ID: 329566 Shyam Kishore Roy, Dhanbad (IN); XP002237627. Abstract & Dzhumauleva, S.A., et al Russ. Satya Niketan Yadav, Dhanbad (IN); J. Phys. Chem. vol. 67, No. 5 (1993) pp. 976–977. Anup Kumar Bandyopadhyay, Dhanbad (IN) Database WPI Section Ch, Week 199627 Derwent Publica tions Ltd., London, GB; Class BO3, AN 1996–266597 (73) ASSignee: Council of Scientific and Industrial XP002237628 & SU 1197396 (Orozhonikidze Chem Pharm Research, New Delhi (IN) Inst) (1995) col. 1, line 52–67, Ex. 2. Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. Primary Examiner Patricia L. Morris (74) Attorney, Agent, or Firm-Ladas & Parry (21) Appl. No.: 10/393,372 (57) ABSTRACT (22) Filed: Mar 20, 2003 The invention provides a process for the manufacture of Int. C.7. - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - C07D 213/56 (51) isonicotinic acid hydrazide(INH) useful in the treatment of (52) - - - - - - - - - - - - 546/316 tuberculosis. The invention relates to the Single Step con (58) Field of Search ......................................... -
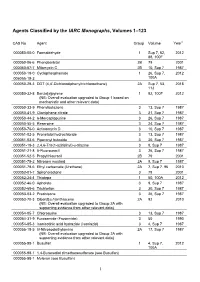
CAS Registry Number Order
Agents Classified by the IARC Monographs, Volumes 1–123 CAS No. Agent Group Volume Year1 000050-00-0 Formaldehyde 1 Sup 7, 62, 2012 88, 100F 000050-06-6 Phenobarbital 2B 79 2001 000050-07-7 Mitomycin C 2B 10, Sup 7 1987 000050-18-0 Cyclophosphamide 1 26, Sup 7, 2012 006055-19-2 100A 000050-29-3 DDT (4,4'-Dichlorodiphenyltrichloroethane) 2A Sup 7, 53, 2018 113 000050-32-8 Benzo[a]pyrene 1 92, 100F 2012 (NB: Overall evaluation upgraded to Group 1 based on mechanistic and other relevant data) 000050-33-9 Phenylbutazone 3 13, Sup 7 1987 000050-41-9 Clomiphene citrate 3 21, Sup 7 1987 000050-44-2 6-Mercaptopurine 3 26, Sup 7 1987 000050-55-5 Reserpine 3 24, Sup 7 1987 000050-76-0 Actinomycin D 3 10, Sup 7 1987 000051-02-5 Pronetalol hydrochloride 3 13, Sup 7 1987 000051-03-6 Piperonyl butoxide 3 30, Sup 7 1987 000051-18-3 2,4,6-Tris(1-aziridinyl)-s-triazine 3 9, Sup 7 1987 000051-21-8 5-Fluorouracil 3 26, Sup 7 1987 000051-52-5 Propylthiouracil 2B 79 2001 000051-75-2 Nitrogen mustard 2A 9, Sup 7 1987 000051-79-6 Ethyl carbamate (Urethane) 2A 7, Sup 7, 96 2010 000052-01-7 Spironolactone 3 79 2001 000052-24-4 Thiotepa 1 50, 100A 2012 000052-46-0 Apholate 3 9, Sup 7 1987 000052-68-6 Trichlorfon 3 30, Sup 7 1987 000053-03-2 Prednisone 3 26, Sup 7 1987 000053-70-3 Dibenz[a,h]anthracene 2A 92 2010 (NB: Overall evaluation upgraded to Group 2A with supporting evidence from other relevant data) 000054-05-7 Chloroquine 3 13, Sup 7 1987 000054-31-9 Furosemide (Frusemide) 3 50 1990 000054-85-3 Isonicotinic acid hydrazide (Isoniazid) 3 4, Sup 7 1987 000055-18-5 N-Nitrosodiethylamine 2A 17, Sup 7 1987 (NB: Overall evaluation upgraded to Group 2A with supporting evidence from other relevant data) 000055-98-1 Busulfan 1 4, Sup 7, 2012 100A 000055-98-1 1,4-Butanediol dimethanesulfonate (see Busulfan) 000055-98-1 Myleran (see Busulfan) 1 Agents Classified by the IARC Monographs, Volumes 1–123 CAS No. -

Isoniazid: a Review of Characteristics, Properties and Analytical Methods
Critical Reviews in Analytical Chemistry ISSN: 1040-8347 (Print) 1547-6510 (Online) Journal homepage: https://www.tandfonline.com/loi/batc20 Isoniazid: A Review of Characteristics, Properties and Analytical Methods Guilherme Felipe dos Santos Fernandes, Hérida Regina Nunes Salgado & Jean Leandro dos Santos To cite this article: Guilherme Felipe dos Santos Fernandes, Hérida Regina Nunes Salgado & Jean Leandro dos Santos (2017) Isoniazid: A Review of Characteristics, Properties and Analytical Methods, Critical Reviews in Analytical Chemistry, 47:4, 298-308, DOI: 10.1080/10408347.2017.1281098 To link to this article: https://doi.org/10.1080/10408347.2017.1281098 Accepted author version posted online: 12 Jan 2017. Published online: 03 Feb 2017. Submit your article to this journal Article views: 356 Citing articles: 6 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=batc20 CRITICAL REVIEWS IN ANALYTICAL CHEMISTRY 2017, VOL. 47, NO. 4, 298–308 http://dx.doi.org/10.1080/10408347.2017.1281098 Isoniazid: A Review of Characteristics, Properties and Analytical Methods Guilherme Felipe dos Santos Fernandes a,b,Herida Regina Nunes Salgadob, and Jean Leandro dos Santosa,b aInstitute of Chemistry, S~ao Paulo State University (UNESP), Araraquara, Brazil; bSchool of Pharmaceutical Sciences, S~ao Paulo State University (UNESP), Araraquara, Brazil ABSTRACT KEYWORDS Isoniazid is a synthetic antimicrobial and one of the most important first-line drugs used in the treatment Analytical methods; high of tuberculosis. Since it was introduced in the therapy in 1952, the drug remains at the front line of the performance liquid antituberculosis treatment mainly due to its potency and high selectivity against Mycobacterium chromatography; isoniazid; tuberculosis. -

Pellagra Encephalopathy Among Tuberculous Patients: Its Relation to Isoniazid Therapy
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.48.7.628 on 1 July 1985. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1985;48:628-634 Pellagra encephalopathy among tuberculous patients: its relation to isoniazid therapy NOBUYOSHI ISHII, YASUO NISHIHARA From the Department of Pathology, University of Occupational and Environmental Health, Kitakyushushi, and the Department of Medicine, Kurate Kyoritsu Hospital, Miyatacho, Fukuokaken, Japan SUMMARY Eight cases of pellagra, diagnosed on the grounds of neuropathological findings and retrospective study of clinical data, were found among 106 necropsy cases of tuberculosis. Although these eight patients had shown various mental, neurological and gastrointestinal symp- toms, as well as skin lesions, the diagnosis of pellagra had not been made clinically. In all the patients, pellagra symptoms appeared during isoniazid therapy. Death occurred 4 to 16 weeks later. Isoniazid inhibits the conversion of tryptophan to niacin and may induce pellagra, particu- larly in poorly nourished patients. Pellagra should be suspected whenever tuberculous patients under treatment with isoniazid develop mental, neurological or gastrointestinal symptoms, even in the absence of typical pellagra dermatitis. Protected by copyright. Pellagra is a disease caused primarily by niacin sented with various mental, neurological, gastro- (nicotinic acid) deficiency and characterised by the intestinal and skin lesions during the course of classical triad of dermatitis, diarrhoea and dementia. isoniazid therapy. Clinically, pellagra had not been A niacin deficiency state can be brought about by suspected in any of them. The aim of this paper is to various causes, such as inadequate food intake, show the clinical and pathological findings of the malabsorption syndrome, increased requirement or eight cases and to call attention to pellagra administration of drugs which interfere with niacin encephalopathy without skin lesions (pellagra sine synthesis. -

The Distribution of Iproniazid in Body Fluids and Tissues
THE DISTRIBUTION OF IPRONIAZID IN BODY FLUIDS AND TISSUES AFTER ORAL ADMINISTRATION AND A METHOD FOR ITS DETERMINATION DISSERTATION Presented In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By CARL EDWARD MOYER, B. S., M. S. <1 The Ohio State University 1959 Approved by Department of Physiological Chemistry ACISOWLEDGSKEHT The author wishes to express his sincere appreciation to Dr. Helen L. Wikoff for her counsel, interest and patience in preparing this work. ii TABLE OP CONTENTS Page Introduction............................................ 1 Historical.............................................. 4 Preparation and Chemical Properties of Iproniazid.... 8 General Methods for the Determination of Carboxylic Acid Derivatives........................... 11 Experimental Procedures and Results................. 14 A. Attempts to Develop Colorimetric Procedures for the Determination of Iproniazid................................... 14 B. Spectrophotometric Procedures............. 16 C. Attempts to Separate Iproniazid from Pyridine Carboxylic Acids and Their Derivatives............................... 19 D. The Determination of Iproniazid in Aqueous Solutions........................................ 2 4 E. Quantitative Estimation of Iproniazid Added to Pooled Serum................ 26 P. Quantitative Estimation of Iproniazid Added to Urine.................................. 32 G. Procedure Finally Adopted for the Quanti tative Determination of Iproniazid In Blood and