Ionic Liquid Mediated Biosynthesis of Gold Nanoparticles Using Elaeis Guineensis (Oil Palm) Leaves Extract
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hdcvibes Vol3 V4
Vol: 3 April 2020 Newsletter VIBES HALAL DEVELOPMENT CORPORATION – THE CUSTODIAN OF MALAYSIA’S HALAL ECONOMY Halal Pharmaceutical Symposium 2020 Signing of MoU between Halal Development Corporation Berhad (HDC) and Acrosx Japan HALAL DEVELOPMENT CORPORATION THE CUSTODIAN OF MALAYSIA’S HALAL ECONOMY Malaysia has long recognized halal as an industry and auspicious driver for value creation and economic growth. The objective to establish Malaysia as a global halal hub was mooted as early as Malaysia’s Vision 2020, followed promptly by the formation of a facilitative industry ecosystem. Early on, Malaysia recognised that the fulfilment of a well-structured halal ecosystem could not manifest in isolation. It has, and continues to rely upon the spirit of harmonious cooperation among public and private sector stakeholders. This is further reflected in Malaysia’s Shared Prosperity Vision 2030 to achieve sustainable and inclusive growth along with fair and equitable distribution across income groups, ethnicities, regions and supply chains. “Halal & Food Hub” is identified as one of the 15 Key Economic Growth Activities (KEGA) derived based on the country’s strengths, capacity and capability, as well as untapped economic potential. The Halal concept has also grown not only as a multi-billion dollar industry, it has evolved in sophistication and application of lifestyle choice. The fact of Halal as being what's permissible in Islamic law has now grown to become what many would describe as a clean and ethical lifestyle choice, among Muslims and those of other religions. Halal practices have proven worthy of choice and gained consumer attraction the world over. However, it's not merely about building markets and selling products, it's about steering the industry in a sustainable trajectory where reach is far more than what we anticipated a decade ago. -

Redstone Commodity Update Q3
Welcome to the Redstone Commodity Update 2020: Q3 Welcome to the Redstone Commodity Moves Update Q3 2020, another quarter in a year that has been strongly defined by the pandemic. Overall recruitment levels across the board are still down, although we have seen some pockets of hiring intent. There appears to be a general acknowledgement across all market segments that growth must still be encouraged and planned for, this has taken the form in some quite senior / structural moves. The types of hires witnessed tend to pre-empt more mid-junior levels hires within the same companies in following quarters, which leaves us predicting a stronger than expected finish to Q4 2020 and start to Q1 2021 than we had previously planned for coming out of Q2. The highest volume of moves tracked fell to the energy markets, notably, within power and gas and not within the traditional oil focused roles, overall, we are starting to see greater progress towards carbon neutrality targets. Banks such as ABN, BNP and SocGen have all reduced / pulled out providing commodity trade finance, we can expect competition for the acquiring of finance lines to heat up in the coming months until either new lenders step into the market or more traditional lenders swallow up much of the market. We must also be aware of the potential impact of the US elections on global trade as countries such as Great Britain and China (amongst others) await the outcome of the impending election. Many national trade strategies and corporate investment strategies will hinge on this result in a way that no previous election has. -

Converting Waste Oil Palm Trees Into a Resource R O G R a M M E P N V I R O N M E N T E
w w w . u n ep. o r g United Nations Environment Programme P.O. Box 30552 Nairobi, 00100 Kenya Tel: (254 20) 7621234 Fax: (254 20) 7623927 E-mail: [email protected] web: www.unep.org CONVERTING WASTE OIL PALM TREES INTO A ESOURCE R ROGRAMME P NVIRONMENT E ATIONS N NITED U Copyright © United Nations Environment Programme, 2012 This publication may be reproduced in whole or in part and in any form for educa- tional or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made. UNEP would appreciate receiv- ing a copy of any publication that uses this publication as a source. No use of this publication may be made for resale or for any other commercial purpose whatsoever without prior permission in writing from the United Nations Environment Programme. Disclaimer The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the United Na- tions Environment Programme concerning the legal status of any country, territory, city or area or of its authorities, or concerning delimitation of its frontiers or boundar- ies. Moreover, the views expressed do not necessarily represent the decision or the stated policy of the United Nations Environment Programme, nor does citing of trade names or commercial processes constitute endorsement. Acknowledgement This document was developed by a team led by Dr. Wan Asma Ibrahim Head of Bioen- ergy Programme, Forest Products Division, Forest Research Institute Malaysia (FRIM) under the overall guidance and supervision of Surya Prakash Chandak, Senior Pro- gramme Officer, International Environmental Technology Centre, Division of Technol- ogy, Industry & Economics, United Nations Environment Programme. -

Of TDM BERHAD’S Annual Report 2019 Is Also Available on Our Website
STRENGTHENING FOCUS, ACHIEVING TRUE POTENTIAL ANNUAL REPORT 2019 STRENGTHENING FOCUS, TH ACHIEVING TRUE POTENTIAL 55 TDM's sustainability effort is derived from the ability to strengthen our focus during challenging ANNUAL times. It is about making critical decisions and improving decision-making for better outcomes GENERAL through the use of robust strategic frameworks. To us, the ever changing macroeconomic challenges MEETING are an opportunity for TDM to make organisational improvements - in our people, our processes, the (AGM) OF technology that we leverage on and the branding of TDM. TDM BERHAD We recognise our ability as a leading player in Broadcast Venue the oil palm plantation industry and demonstrate Tricor Leadership Room continuous growth in our healthcare business. We believe the unrelenting efforts in optimising our Unit 32-01, Level 32, Tower A strength will lead TDM to the path of achieving its Vertical Business Suite, Avenue 3 true potential. Bangsar South No. 8 Jalan Kerinchi 59200 Kuala Lumpur. VISION CORE VALUES Date & Time To be the iconic corporation Monday, 27 July 2020 of the East Coast that creates GOOD 11.00 a.m. GOVERNANCE sustainable values for our stakeholders. TEAM WORK MISSION To be a model corporate citizen in PEOPLE TDM BERHAD ANNUAL REPORT CENTRIC 2019 DIGITAL VERSION Terengganu; • To create sustainable value for our Follow the steps below to scan the shareholders. QR Code reader in 3 easy steps INNOVATIVE • To improve the well being of our Download the “QR CodeReader” on App Store or stakeholders while protecting the Google Play. environment. • To deliver quality products & ENVIRONMENTAL Run the QR Code Reader app FRIENDLY and point your camera to the services above expectation for QR Code. -

Government Transformation Programme
Government Transformation Programme JABATAN PERDANA MENTERI Annual Report 2010 2010 marked the introduction and implementation of Malaysia’s Government Transformation Programme (GTP) and a new chapter in our young nation’s history. This bold and unprecedented programme aimed to radically transform the way the Government worked so we could better serve the rakyat, regardless of race, religion or social status. In embracing change, we learnt how to listen more effectively, speak more openly, see things for what they really are, develop a positive course of action and deliver tangible solutions. These efforts have laid strong foundations for the future progress of our nation and given the rakyat the assurance of a better future. This inaugural annual report of the GTP serves as a narrative of all that transpired in 2010. It records our many successes as well as shortcomings, outlines the key lessons learnt and the next steps we will take to achieve Vision 2020. As the GTP continues to impact upon and transform the lives of the rakyat for the better, the Government will continue to learn from its achievements and limitations, set higher goals and dream bigger dreams. CONTENTS Perspectives from the Top 2 – Progress of the Government Transformation Programme The Year Things Changed 4 – An Overview of Year 1, Horizon 1 of the GTP 6 Big Results Fast 14 Overview of the Government Transformation Programme 26 2010 Results of the Six National Key Result Areas 27 I Reducing Crime 65 I Fighting Corruption 105 I Improving Student Outcomes 131 I Raising -

Eleventh Malaysia Plan 2016-2020 Anchoring GRowth on People
ELEVENTH MALAYSIA PLAN 2016-2020 ANCHORING G ROWTH ON PEOPLE ISBN 978-9675842085 For further information refer to: Director General, Economic Planning Unit, Prime Minister’s Department, Block B5 & B6, Federal Government Administrative Centre, 62502 Putrajaya. MALAYSIA. http://www.epu.gov.my email: [email protected] Tel.: 603-8000 8000 Fax: 603-8888 3755 Released on 21st May 2015 Reprinted on 29th May 2015 Publisher’s Copyright© All rights reserved. No part of this publication may be reproduced, copied, stored in any retrieval system or transmitted in any form or by any means – electronic, mechanical, photocopying, recording or otherwise; without prior permission of Economic Planning Unit, Prime Minister’s Department, Malaysia. Printed by Percetakan Nasional Malaysia Berhad, Kuala Lumpur, 2015 www.printnasional.com.my Email: [email protected] Tel: 03-92366895 Fax: 03-92224773 ELEVENTH MALAYSIA PLAN 2016-2020 ANCHORING G ROWTH ON PEOPLE Foreword Dato’ Sri Mohd Najib bin Tun Haji Abdul Razak Prime Minister of Malaysia i The Eleventh Malaysia Plan, 2016-2020, marks a momentous milestone in our nation’s history. With 2020 now just five years away, the Eleventh Plan is the next critical step in our journey to become an advanced nation that is inclusive and sustainable. In the last five years, although Malaysia encountered headwinds from a global economic slowdown, our economy has done extremely well with GDP growth among the fastest in the region. The quality of life of the rakyat has also improved as reflected by the increase in both per capita income and the average household income. This was made possible by the numerous reforms that were put in place by the Government to improve the quality of life of the people. -
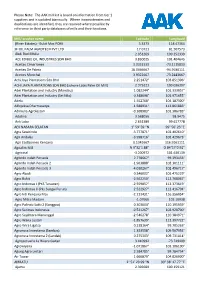
The AAK Mill List Is Based on Information from Tier 1 Suppliers and Is Updated Biannually
Please Note: The AAK mill list is based on information from tier 1 suppliers and is updated biannually. Where inconsistencies and duplications are identified, they are resolved where possible by reference to third party databases of mills and their locations. Mill/ crusher name Latitude Longitude (River Estates) - Bukit Mas POM 5.3373 118.47364 3F OIL PALM AGROTECH PVT LTD 17.0721 81.507573 Abdi Budi Mulia 2.051269 100.252339 ACE EDIBLE OIL INDUSTRIES SDN BHD 3.830025 101.404645 Aceites Cimarrones 3.0352333 -73.1115833 Aceites De Palma 18.0466667 -94.9186111 Aceites Morichal 3.9322667 -73.2443667 Achi Jaya Plantations Sdn Bhd 2.251472° 103.051306° ACHI JAYA PLANTATIONS SDN BHD (Johore Labis Palm Oil Mill) 2.375221 103.036397 Adei Plantation and Industry (Mandau) 1.082244° 101.333057° Adei Plantation and Industry (Sei Nilo) 0.348098° 101.971655° Adela 1.552768° 104.187300° Adhyaksa Dharmasatya -1.588931° 112.861883° Adimulia Agrolestari -0.108983° 101.386783° Adolina 3.568056 98.9475 Aek Loba 2.651389 99.617778 AEK NABARA SELATAN 1° 59' 59 "N 99° 56' 23 "E Agra Sawitindo -3.777871° 102.402610° Agri Andalas -3.998716° 102.429673° Agri Eastborneo Kencana 0.1341667 116.9161111 Agrialim Mill N 9°32´1.88" O 84°17´0.92" Agricinal -3.200972 101.630139 Agrindo Indah Persada 2.778667° 99.393433° Agrindo Indah Persada 2 -1.963888° 102.301111° Agrindo Indah Persada 3 -4.010267° 102.496717° Agro Abadi 0.346002° 101.475229° Agro Bukit -2.562250° 112.768067° Agro Indomas I (PKS Terawan) -2.559857° 112.373619° Agro Indomas II (Pks Sungai Purun) -2.522927° -

GOVERNANCE CONVENTION 2019 Rising Beyond Principles and Policies
IIC-SIDC GOVERNANCE CONVENTION 2019 Rising Beyond Principles and Policies 18-19 NOVEMBER 2019 UDED Royale Chulan Kuala Lumpur SPEAKERS & MODERATORS - DAY 1 SPECIAL SESSION TAN SRI RAFIDAH AZIZ Tan Sri Rafidah Aziz was born in Selama, Perak on 4 November 1943 and had her primary, secondary, and tertiary education in various schools in Kuala Lumpur, Kota Bharu and Johor Bharu. She qualified with a BA Degree in 1966, and Masters in Economics in 1970, from the University of Malaya. She worked as Tutor and as Lecturer in the Faculty of Economics, University of Malaya between 1966 and 1976. She was appointed as Senator in 1974 and resigned to contest in the General Elections in 1978. She served as Member of Parliament from 1978 to 2013 in the Selayang Constituency (1978 to 1982) and Kuala Kangsar Constituency (1982 – 2013) In 1976, she was appointed as Parliamentary Secretary in the Ministry of Public Enterprises and in 1977 promoted to Deputy Minister of Finance. In 1980, she was made Minister of Public Enterprises a post she held for 7 years. In 1987, she was appointed Minister for Trade and Industry (subsequently redesignated Minister of International Trade and Industry), and served for 21 years up to 2008. She served in the UMNO Supreme Council for 38 years since winning a seat in the council in 1975. She has received various award from the States of Selangor, Perak, Malacca, Terengganu and Sarawak, as well as awards from Thailand, Argentina and Chile. She has also been conferred Honorary Doctorates from University Malaya, University Putra Malaysia, University Utara Malaysia, University Tun Abdul Razak Malaysia, HELP University, Cyberjaya University College, University Tunku Abdul Rahman and Dominican University of California, United States. -

Ioi Pan-Century Edible Oils V2.Pdf
IOI Pan-Century Edible Oils List of suppliers Reporting period: Jan - Dec 2018 Certification Parent Company Supplier Name RSPO ISCC ISPO Latitude Longitude YPJ Oil Palm Estate Sdn Bhd Alaf Palm Oil Mill 1.71890 103.78220 Vila Sutera Sdn Bhd Kilang Kelapa Sawit Bukit Kapah 5.10930 102.89730 Victory Enghoe Plantations Sdn Bhd Victory Eng Hoe 1.78910 103.36410 Veetar Palm Oil Mill Sdnbhd Veetar Palm Oil Mill Sdn Bhd 5.33660 116.94320 United Malacca Bhd Meridian Plantations Sdn Bhd 6.45480 117.43120 Syarikat Penanaman Bukit Senorang Sdn United Malacca Bhd 3.46930 102.31650 Bhd Tradewinds Plantation Bhd Melur Gemilang Palm Oil Mill Sdn Bhd 1.20970 110.81400 Tradewinds Plantation Berhad Batu Putih 5.18720 118.44140 Tradewinds Plantation Berhad Sungai Kachur Palm Oil Mill 1.78330 103.76270 Tradewinds Plantation Berhad Trusan Pom 4.82860 115.26510 Tradewinds Plantation Berhad Ulu Sebol Palm Oil Mill 1.88910 103.62260 Topaz Emas Sdn Bhd Topaz Emas Sdn Bhd 4.55337 100.72600 The China Engineers (M) Sdn Bhd Tian Siang Oil Mill (Pahang) Sdn Bhd 3.80352 101.84205 TH Plantations Berhad Kilang Sawit Bukit Lawiang 1.96570 103.43410 TH Plantations Berhad Kilang Sawit Kota Bahagia 2.98650 102.92700 Tee Teh Palm Oil Mill Tee Teh Palm Oil Mill 2.84120 102.86560 TDM Plantation Sdn Bhd Kemaman Palm Oil Mill IP 4.40300 103.24800 TDM Plantation Sdn Bhd Sungai Tong Palm Oil Mill MB 5.33880 102.85980 Tanjung Panjang Palm Oil Mill Sdn Bhd Tanjong Malim MB X 3.69430 101.48580 Tanjung Panjang Palm Oil Mill Sdn Bhd Tanjung Panjang Palm Oil Mill Sdn Bhd 5.64430 118.33310 Sungai Terah Palm Oil Mill Sdn Bhd Sungai Terah Palm Oil Mill Sdn Bhd 4.97640 101.95440 Sukau Palm Oil Mill Sdn Bhd Sukau Palm Oil Mill 5.56890 118.20300 Suburban Properties Sdn. -

Palm Oil Production and Opportunities for Finnish Technology and Know-How Transfer
Lappeenranta University of Technology Faculty of Technology, LUT Energy Research Report 1 SUSTAINABILITY OF PALM OIL PRODUCTION AND OPPORTUNITIES FOR FINNISH TECHNOLOGY AND KNOW-HOW TRANSFER Virgilio Panapanaan Tuomas Helin Marjukka Kujanpää Risto Soukka Jussi Heinimö Lassi Linnanen 2009 ISBN 978-952-214-737-0 ISBN 978-952-214-758-5(PDF) ISSN 1798-1328 Copyright © Lappeenranta University of Technology, 2009 PUBLISHER Lappeenranta University of Technology Skinnarilankatu 34, P.O. Box 20, FI-53851 Lappeenranta, Finland Tel. +358 5 621 11, fax. +358 5 621 2350 This publication is available in PDF format on the Internet at www.doria.fi/lutpub Photos: 1. Oil palm plantation by: Rhett A. Butler, mongabay.com 2. Oil palm fruit by: Rhett A. Butler, mongabay.com 3. Wastewater aerobic treatment tank: Mika Horttanainen, LUT 4. Improved and modern dryer: Mika Horttanainen, LUT 2 ABSTRACT Virgilio Panapanaan, Tuomas Helin, Marjukka Kujanpää, Risto Soukka, Jussi Heinimö and Lassi Linnanen Sustainability of palm oil production and opportunities for Finnish technology and know- how transfer Lappeenranta University of Technology Institute of Energy Technology Research Report 1 March 2009 95 pages, 18 figures, 14 tables Keywords: palm oil, bio-diesel, CDM projects, carbon footprint, greenhouse gas balance, life cycle assessment The global demand for palm oil is growing, thus prompting an increase in the global production particularly in Malaysia and Indonesia. Such increasing demand for palm oil is due to palm oil’s relatively cheap price and versatile advantage both in edible and non-edible applications. Along with the increasing demand for palm oil, particularly for the production of biofuel, is a heated debate on its sustainability. -

Malaysian Agricultural Transformation
AGRICULTURE AND FOOD GLOBAL PRACTICE & POVERTY AND EQUITY GLOBAL PRACTICE Public Disclosure Authorized NOVEMBER 2019 THE MALAYSIA DEVELOPMENT EXPERIENCE SERIES Agricultural Transformation and Public Disclosure Authorized Inclusive Growth The Malaysian Experience Public Disclosure Authorized Public Disclosure Authorized CONNECT WITH US wbg.org/Malaysia @WorldBankMalaysia @WB_AsiaPacific http://bit.ly/WB_blogsMY NOVEMBER 2019 THE MALAYSIA DEVELOPMENT EXPERIENCE SERIES Agricultural Transformation and Inclusive Growth The Malaysian Experience AGRICULTURE AND FOOD GLOBAL PRACTICE & POVERTY AND EQUITY GLOBAL PRACTICE About KNOWLEDGE & RESEARCH The World Bank Group’s current partnership with Malaysia is focused on knowledge sharing. It is centered on support for Malaysia’s vision to join the ranks of high-income and developed economies through inclusive and sustainable growth, and to share its lessons with developing countries. In March 2016, the World Bank Group officially launched its Global Knowledge and Research Hub (the Hub) in Malaysia. The Hub is the first of its kind, serving both as a field presence in Malaysia and as a global knowledge and research hub. It focuses on sharing Malaysia’s people- centered development expertise and creating new innovative policy research on local, regional, and global issues. Knowledge & Research reports are flagship work emanating from the teams based in the Malaysia Hub. The Malaysia Development Experience Series captures key lessons from Malaysia relevant for emerging economies in Asia, Africa, and elsewhere that are transitioning out of poverty and into shared prosperity. Cover Photos attribution: © bigstockphoto.com The findings, interpretations, and conclusions expressed in this report do not necessarily reflect the views of the Executive Directors of the World Bank or the governments they represent. -

Appendix Tables
APPENDIX TABLES Malaysia WT/TPR/S/225 Page 75 Table AI.1 Merchandise exports by product group, 2004-08 (US$ million and per cent) 2004 2005 2006 2007 2008 Total exports (US$ million) 126,639.7 141,624.0 160,669.2 176,205.6 198,846.4 (Per cent of total) Total primary products 23.1 23.9 24.8 27.5 34.0 Agriculture 10.4 9.4 9.7 11.6 14.0 Food 7.9 6.9 7.0 9.2 11.6 4222 Palm oil, fractions 3.8 3.0 3.2 4.7 6.4 4312 Fats/oils, partly or wholly hydrogenated, etc. 0.7 0.7 0.6 0.8 0.9 Agricultural raw material 2.5 2.5 2.7 2.4 2.4 2312 Natural rubber excluding latex 1.0 1.0 1.3 1.1 1.2 Mining 12.7 14.5 15.1 15.8 20.0 Ores and other minerals 0.2 0.1 0.2 0.2 0.3 Non-ferrous metals 1.0 1.0 1.2 1.4 1.5 Fuels 11.6 13.4 13.7 14.2 18.2 3330 Crude oils of petroleum and bituminous minerals 4.8 5.7 5.5 5.5 6.6 3431 Natural gas, liquefied 3.6 4.0 4.0 4.3 6.1 Manufactures 75.5 74.5 73.4 70.9 54.2 Iron and steel 1.5 1.3 1.6 1.7 1.6 Chemicals 5.6 5.8 5.4 6.0 5.9 Other semi-manufactures 4.3 4.0 4.5 4.8 4.9 6343 Plywood of sheets <6 mm thickness 1.1 1.0 1.2 1.0 0.9 Machinery and transport equipment 54.5 54.0 52.6 49.0 33.2 Power generating machines 0.4 0.4 0.3 0.3 0.2 Other non-electrical machinery 2.7 2.7 2.8 3.1 2.9 Office machines & telecommunication equipment 44.4 42.6 42.2 38.8 24.0 7599 Parts and accessories of 751.1, 751.2, 751.9, and 752 6.8 6.2 6.9 6.0 5.5 7522 Digital automatic data processing machines 4.5 4.8 4.5 4.1 2.9 7529 Data processing equipment, n.e.s.