Who's Who in Biotech
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NSABB June-July 2005 Meeting Agenda
First Meeting of the National Science Advisory Board for Biosecurity June 30 – July 1, 2005 Hyatt Regency Bethesda 7400 Wisconsin Ave. Bethesda, Maryland, 20814 USA Hotel Phone: 301-657-1234 Agenda Webcast: To watch the live webcast of the meeting, click here. The webcast can only be viewed when the meeting is in session at 8:00am-6:00pm on June 30 and at 8:00am-1:30pm on July 1 Eastern Time. You will need RealOne Player to view the webcast. If you do not already have RealOne Player on your computer, download here. Presentation slides: To access the following PowerPoint presentations, click on the presentation titles below. June 30, 2005 Opening Remarks and Swearing in Ceremony Elias Zerhouni, M.D. Director of the National Institutes of Health (NIH) Chair's Remarks and Agenda Overview Dennis L. Kasper, M.D. NSABB Chair Harvard Medical School Introduction of NSABB Members NSABB Structure and Operations Thomas Holohan, M.D. NSABB Executive Director, NIH Office of Biotechnology Activities Break Perspectives on Biosecurity in the Life Sciences NSABB Voting Members Impetus for NSABB: Enhancing Biosecurity on the Life Sciences Rajeev Venkayya, M.D. Special Assistant to the President, Senior Director for Biological and Chemical Defense White House Homeland Security Council Perspectives on Biosecurity in the Life Sciences NSABB Ex Officio Members Lunch Session I- The Development of Criteria for Identifying Dual Use Research and Research Results Introduction: Issues of Relevance to Criteria Development Arturo Casadevall, M.D., Ph.D. Professor of Medicine and of Microbiology & Immunology and Chief of Infectious Diseases Albert Einstein College of Medicine National Research Council Perspective: Experiments of Concern Ron Atlas, Ph.D. -

From the Academy
FROM THE ACADEMY Joint American Academy of DermatologyeNational Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy Craig A. Elmets, MD (Co-Chair),a HenryW.Lim,MD,b Benjamin Stoff, MD, MA,c Cody Connor, MD,a Kelly M. Cordoro, MD,d Mark Lebwohl, MD,e AprilW.Armstrong,MD,MPH,f Dawn M. R. Davis, MD,g Boni E. Elewski, MD,a Joel M. Gelfand, MD, MSCE,h Kenneth B. Gordon, MD,i AliceB.Gottlieb,MD,PhD,j Daniel H. Kaplan, MD, PhD,k Arthur Kavanaugh, MD,l Matthew Kiselica, BA/BS,m Dario Kivelevitch, MD,n Neil J. Korman, MD, PhD,o Daniela Kroshinsky, MD, MPH,p Craig L. Leonardi, MD,q Jason Lichten, MD,m NehalN.Mehta,MD,MSCE,r Amy S. Paller, MD,s Sylvia L. Parra, MD,t Arun L. Pathy, MD,u Elizabeth A. Farley Prater, MD,v Reena N. Rupani, MD,e Michael Siegel, PhD,m BruceE.Strober,MD,PhD,w,x Emily B. Wong, MD,y Jashin J. Wu, MD,z Vidhya Hariharan, PhD,aa and Alan Menter, MD (Co-Chair)n Birmingham, Alabama; Detroit, Michigan; Atlanta, Georgia; San Francisco, Los Angeles, San Diego, and Irvine, California; New York, New York; Rochester, Minnesota; Philadelphia and Pittsburgh, Pennsylva- nia; Milwaukee, Wisconsin; Portland, Oregon; Dallas and San Antonio, Texas; Cleveland, Ohio; Boston, Massachusetts; St. Louis, Missouri; Bethesda, Maryland; Chicago and Rosemont, Illinois; Sumter, South Carolina; Centennial, Colorado; Oklahoma City, Oklahoma; Farmington, Connecticut; and Waterloo, Ontario, Canada Psoriasis is a chronic inflammatory disease involving multiple organ systems and affecting approximately 3.2% of the world’s population. -

Como Citar Este Artigo Número Completo Mais Informações Do
Encontros Bibli: revista eletrônica de biblioteconomia e ciência da informação ISSN: 1518-2924 Programa de Pós-graduação em Ciência da Informação - Universidade Federal de Santa Catarina STANFORD, Jailiny Fernanda Silva; SILVA, Fábio Mascarenhas e Prêmio Nobel como fator de influência nas citações dos pesquisadores: uma análise dos laureados de Química e Física (2005 - 2015) Encontros Bibli: revista eletrônica de biblioteconomia e ciência da informação, vol. 26, e73786, 2021, Janeiro-Abril Programa de Pós-graduação em Ciência da Informação - Universidade Federal de Santa Catarina DOI: https://doi.org/10.5007/1518-2924.2021.e73786 Disponível em: https://www.redalyc.org/articulo.oa?id=14768130002 Como citar este artigo Número completo Sistema de Informação Científica Redalyc Mais informações do artigo Rede de Revistas Científicas da América Latina e do Caribe, Espanha e Portugal Site da revista em redalyc.org Sem fins lucrativos acadêmica projeto, desenvolvido no âmbito da iniciativa acesso aberto Artigo Original Prêmio Nobel como fator de influência nas citações dos pesquisadores: uma análise dos laureados de Química e Física (2005 - 2015) Nobel Prize as an influencing factor in researchers' citations: an analysis of Chemistry and Physics laureates (2005 to 2015) Jailiny Fernanda Silva STANFORD Mestre em Ciência da Informação (PPGCI/UFPE) Bibliotecária-chefe Seminário Teológico Batista do Norte do Brasil (STBNB), Recife, Brasil [email protected] https://orcid.org/0000-0003-2112-6561 Fábio Mascarenhas e SILVA Doutor em Ciência da Informação (USP), Professor Associado Universidade Federal de Pernambuco, Departamento de Ciência da Informação, Recife, Brasil [email protected] https://orcid.org/0000-0001-5566-5120 A lista completa com informações dos autores está no final do artigo RESUMO Objetivo: Analisa a influência nos índices de citação por parte dos pesquisadores que foram contemplados pelo prêmio Nobel nas áreas da Física e Química no período de 2005 a 2015. -

Biochemistrystanford00kornrich.Pdf
University of California Berkeley Regional Oral History Office University of California The Bancroft Library Berkeley, California Program in the History of the Biosciences and Biotechnology Arthur Kornberg, M.D. BIOCHEMISTRY AT STANFORD, BIOTECHNOLOGY AT DNAX With an Introduction by Joshua Lederberg Interviews Conducted by Sally Smith Hughes, Ph.D. in 1997 Copyright 1998 by The Regents of the University of California Since 1954 the Regional Oral History Office has been interviewing leading participants in or well-placed witnesses to major events in the development of Northern California, the West, and the Nation. Oral history is a method of collecting historical information through tape-recorded interviews between a narrator with firsthand knowledge of historically significant events and a well- informed interviewer, with the goal of preserving substantive additions to the historical record. The tape recording is transcribed, lightly edited for continuity and clarity, and reviewed by the interviewee. The corrected manuscript is indexed, bound with photographs and illustrative materials, and placed in The Bancroft Library at the University of California, Berkeley, and in other research collections for scholarly use. Because it is primary material, oral history is not intended to present the final, verified, or complete narrative of events. It is a spoken account, offered by the interviewee in response to questioning, and as such it is reflective, partisan, deeply involved, and irreplaceable. ************************************ All uses of this manuscript are covered by a legal agreement between The Regents of the University of California and Arthur Kornberg, M.D., dated June 18, 1997. The manuscript is thereby made available for research purposes. All literary rights in the manuscript, including the right to publish, are reserved to The Bancroft Library of the University of California, Berkeley. -

Annual Report on Annual Reports 2016
ANNUAL REPORT ON ANNUAL REPORTS 2016 TOP 400 ANNUAL REPORTS WHO RANKS WHERE? 100 ANNUALS IN BRIEF BEST REPORTING PRACTICES Company Value > Report Value Annual Report on Annual Reports 2016 Contents Report rating scale 3 Top 400 annual reports 4 Who ranks where? 25 From ABB to ZTE 100 annuals in brief 58 From Abbott to Yamaha How important is the annual report today? 92 Views from Cecilia Ketels, Kellie Friery, Renee Carter, David Robinson, Kaevan Gazdar, Elena Moskvina, Thomas Rosenmayr, Rob Stangroom, Andrey Kozhevnikov, Ana Santamarina, Katie Holcomb, Ananda Jagoda Best practices on key report attibutes 100 Strategy, message, investor information, risks, style, online… How we make it 121 How is your report doing? The report scan 127 The report rating panel 128 Robert Berick, Susan Blesener, Renee Carter, Vero Escarmelle, Helena Fournial, Kaevan Gazdar, Mike Guillaume, Pradip Seth, Eva Wolosiuk Making reports pay off 133 e.com – ReportWatch 135 2 Report rating scale A+ ééééé First-rate A éééé(é) Excellent A- éééé Very good B+ ééé(é) Sound B ééé Average B- éé(é) Uneven C+ éé Common C é(é) Substandard C- é Poor D (é) Uncompetitive 3 Top 400 annual reports AkzoNobel (No. 1) Electrolux (No. 2 ) SCA (No. 3) Volvo (No. 4) 4 Report rank Company Country Report rating Compare 1 AKZONOBEL Netherlands A+ DUPONT 2 ELECTROLUX Sweden A+ WHIRLPOOL 3 SCA Sweden A+ KIMBERLY-CLARK 4 VOLVO Sweden A+ DAIMLER 5 POTASHCORP Canada A+ AGRIUM 6 ATLAS COPCO Sweden A SANDVIK 7 STORA ENSO Finland A UPM 8 BOLIDEN Sweden A GLENCORE 9 WIENERBERGER Austria A BORAL 10 -

Biotechnology Worldwide
Biotechnology Worldwide There are several countries that are making special efforts to both develop and capitalise on Biotechnology. Chief amongst them is America, though cutting edge work is also going on in the UK, Ireland, Germany, Korea, Singapore, China and Japan. • America is the world leader in biotechnology, it has 1,379 biotechnology companies and employs 174,000 people. It spends £9 billion on research into biotechnology. • The European market for goods and services dependent on biotechnology is currently estimated at £30 billion and is forecast to exceed £100 billion by the year 2005 • The UK leads Europe in biotechnology and employs 19,000 people • The UK has 300 dedicated biotechnology companies and a further 250-300 involved in broader bioscience related activities • The industrial sectors which stand to benefit from biotechnology are pharmaceutical, agriculture, food and drink, chemicals and environmental technologies • Germany is the second strongest country in Europe, with 332 companies but fewer products in development than the UK. UK The UK biotechnology industry is regarded as second only to the huge effort taking place in the States. UK biotechnology companies generate over a billion pounds in revenue; half of this is pumped back into research and development. The industry has particular strengths, for example: • Britain was a key player in the world wide project of sequencing the 30,000 genes of the human genome. The announcement of the first working draft of the human genome marks a significant step forward in our understanding of the way in which we understand and develop treatments for incurable genetic conditions. -

Hd Model of a Conscious Cosmos
J. Nonlocality: Special Issue on Psi and Nonlocal Mind, 2017 ISSN: 2167-6283 Quantum semiotics Stephen Jarosek E-mail: [email protected] Submitted: November 22, 2016 …what we call matter is not completely dead, but is merely mind hidebound with habits. It still retains the element of diversification; and in that diversification there is life. Charles Sanders Peirce, CP 6.158 (1931-1966)1 It has become fashionable, these days, to incorporate the word quantum into a title, whenever someone wants to sell a book or an article, on topics ranging from home cooking to auto repair. Far from entertaining such indulgences, in quantum semiotics, we are interested in the question of whether the principles of consciousness might somehow be relevant to the realm of the very small. This relates to panpsychism. To some, panpsychism is also a four-letter word that carries its own baggage. We need to move past this, with humility, and certainly at least in the spirit of brainstorming. There is “something” going on that now has some of our most enquiring minds contemplating whether we are not in fact just players in a matrix illusion, a kind of computer simulation. We don’t need to resort to such conspiracy theories, just yet, but we do need to keep an open mind. The word quantum relates to discreteness as opposed to continuum. Matter is comprised of discrete atoms and molecules and subatomic particles… electrons occupy energy levels in atoms in discrete jumps… we have the wave-particle duality of discrete photons as packets of energy… and thus we have Planck’s constant that plays an integral part in the quantum narrative. -

Unique Epitopes on Cεmx in Ige–B Cell Receptors Are Potentially Applicable for Targeting Ige-Committed B Cells
Unique Epitopes on CεmX in IgE−B Cell Receptors Are Potentially Applicable for Targeting IgE-Committed B Cells This information is current as Jiun-Bo Chen, Pheidias C. Wu, Alfur Fu-Hsin Hung, of October 1, 2021. Chia-Yu Chu, Tsen-Fang Tsai, Hui-Ming Yu, Hwan-You Chang and Tse Wen Chang J Immunol 2010; 184:1748-1756; Prepublished online 18 January 2010; doi: 10.4049/jimmunol.0902437 http://www.jimmunol.org/content/184/4/1748 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2010/01/13/jimmunol.090243 Material 7.DC1 http://www.jimmunol.org/ References This article cites 52 articles, 19 of which you can access for free at: http://www.jimmunol.org/content/184/4/1748.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on October 1, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2010 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Unique Epitopes on C«mX in IgE–B Cell Receptors Are Potentially Applicable for Targeting IgE-Committed B Cells Jiun-Bo Chen,*,† Pheidias C. -

Biotechnology and Society Syllabus
Nanyang Technological University Humanities and Social Sciences Semester 1, AY2017-2018 HH3010 Biotechnology and Society Syllabus Subject Description This subject will introduce students to selected research and commercial applications of modern biotechnology in order to discuss the broader social, ethical, risk, and regulatory issues that arise from them. A range of topics will be covered in this subject, including genetic engineering, cloning, stem cell research, the production of pharmaceuticals, the human genome project, genetic testing, assisted reproductive technologies, and synthetic biology. Students will consider debates that have taken place in the wider community about ownership, commercialisation, identity, governance, animal welfare, human well-being, and expertise in relation to these applications of modern biotechnology. Prerequisites: Nil Academic Units: 3 Teaching Staff Hallam Stevens Office: HSS-05-07 Email: [email protected] Attendance Requirements Students are expected to attend one two hour lecture and one one-hour tutorial once per week: Lectures: Wednesdays 12.30-2.30pm (LHS Lecture Theatre) Tutorials: Wednesdays 3.30pm-4.30pm OR 4.30pm-5.30pm OR 5.30-6.30pm (LHS TR+45) Tutorials begin in Week 2 and run through to Week 13 (except for weeks 7 and 9). You are required to attend at least eight of these tutorials. If you attend fewer than 8 tutorials you will get zero for your participation and discussion grade 1 (20% of your overall grade). This includes any excused absences (eg. medical reasons still count as “missed” tutorials). Medical certificates are not a get out of jail free card. Missing a seminar without an MC will mean an automatic zero for any attendance and participation marks awarded for that week. -

Biotechnology and the Economics of Discovery in the Pharmaceutical Industry
BIOTECHNOLOGY AND THE ECONOMICS OF DISCOVERY IN THE PHARMACEUTICAL INDUSTRY HELEN SIMPSON Office of Health Economics 12 Whitehall London SWlA 2DY ©October 1998. Office of Health Economics. Price £7.50 ISBN 1 899040 60 9 Printed by BSC Print Ltd, London. About the Author Helen Simpson is currently a researc~ economist at the Institute for Fiscal Studies and was formerly an economist at the Department of Trade and Industry. However, the opinions expressed here are her own and do not necessarily reflect the views of the IFS or of DTI officials or ministers. Acknowledgements This paper has been developed from my MPhil Economics thesis Scientist Entrepreneurs and the Finance of Biotech Companies. I would like to thank Margaret Meyer, Paul David and Gervas Huxley for their valuable suggestions. I am particularly grateful to Hannah Kettler and Jon Sussex for their advice and editorial inputs to the paper. My thanks also go to Adrian Towse and members of the OHE Editorial Board for their comments, and to the following individuals who gave me their insights into the pharmaceutical industry: Dr Trevor Jones, Director General, ABPI; Dr Janet Dewdney, Chairman, Adprotech; Dr Clive Halliday, Head of Global External Scientific Affairs, Glaxo Wellcome; Mr Alan Galloway, Head of Research Administration, Dr Nick Scott-Ram, Director of Corporate Affairs, and Dr Philip Huxley, all of British Biotech; Christine Soden, Finance Director, Chiroscience; Ian Smith, Lehman Brothers Pharmaceutical Research; and Paul Murray, 31. The Office of Health Economics Terms of Reference The Office of Health Economics (OHE) was founded in 1962. Its terms of reference are to: • commission and undertake research on the economics of health and health care; • collect and analyse health and health care data from the UK and other countries; • disseminate the results of this work and stimulate discussion of them and their policy implications. -

Michael S. Brown, MD
DISTINGUISHED PHYSICIANS AND Michael S. Brown, M.D. Sir Richard Roberts, Ph.D. Winner, 1985 Nobel Prize in Physiology or Medicine Winner, 1993 Nobel Prize in Physiology or Medicine MEDICAL SCIENTISTS MENTORING Winner, 1988 Presidential National Medal of Science A globally prominent biochemist and molecular biologist, DELEGATES HAVE INCLUDED... Dr. Brown received the world’s most prestigious medical Dr. Roberts was awarded the Nobel Prize for his prize for his work describing the regulation of the groundbreaking contribution to discovering RNA splicing. cholesterol metabolism. His work laid the foundation for Dr. Roberts is dedicating his future research to GMO crops the class of drugs now called statins taken daily by more than 20 million and food sources, and demonstrating the effect they have on humanity. — GRANDg MASTERS — people worldwide. Ferid Murad, M.D., Ph.D. Mario Capecchi, Ph.D. Boris D. Lushniak, M.D., M.P.H Winner, 1998 Nobel Prize in Physiology or Medicine Academy Science Director The Surgeon General of the United States (acting, 2013-2014) Winner, 2007 Nobel Prize in Physiology or Medicine A world-renowned pioneer in biochemistry, Dr. Murad’s Winner, 2001 National Medal of Science Rear Admiral Lushniak, M.D., M.P.H., was the United award-winning research demonstrated that nitroglycerin Winner, 2001 Lasker Award States’ leading spokesperson on matters of public health, and related drugs help patients with heart conditions by Winner, 2003 Wolf Prize in Medicine overseeing the operations of the U.S. Public Health Service releasing nitric oxide into the body, thus relaxing smooth Mario Capecchi, Ph.D., a biophysicist, is a Distinguished Commissioned Corps, which consists of approximately muscles by elevating intracellular cyclic GMP, leading to vasodilation and Professor of Human Genetics at the University of Utah School of Medicine. -
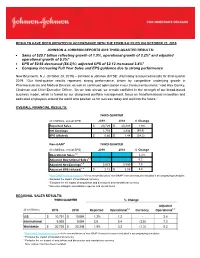
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures