QTL Analysis Reveals Genomic Variants Linked to High-Temperature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Whole Genome Sequencing Identifies Novel Structural Variant in a Large Indian Family Affected with X - Linked Agammaglobulinemia
medRxiv preprint doi: https://doi.org/10.1101/2020.10.05.20200949; this version posted October 6, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-ND 4.0 International license . Whole Genome Sequencing identifies novel structural variant in a large Indian family affected with X - linked agammaglobulinemia 1,2,# 3,5,#,$ 3,4 3 Abhinav Jain , Geeta Madathil Govindaraj , Athulya Edavazhippurath , Nabeel Faisal , Rahul C 1 1,2 6 3 1 1 Bhoyar , Vishu Gupta , Ramya Uppuluri , Shiny Padinjare Manakkad , Atul Kashyap , Anoop Kumar , 1,2 1,2 7 7 3 Mohit Kumar Divakar , Mohamed Imran , Sneha Sawant , Aparna Dalvi , Krishnan Chakyar , 7 6 1,2,$ 1,2,$ Manisha Madkaikar , Revathi Raj , Sridhar Sivasubbu , Vinod Scaria 1 CSIR-Institute of Genomics and Integrative Biology, Mathura Road, New Delhi, Delhi, India 2 Academy of Scientific and Innovative Research (AcSIR), Mathura Road, New Delhi, Delhi, India 3 Department of Pediatrics, Government Medical College Kozhikode, Kozhikode, Kerala, India 4 Multidisciplinary Research Unit, Government College Kozhikode, Kozhikode, Kerala, India 5 FPID Regional Diagnostic Centre, Government Medical College Kozhikode, Kozhikode, Kerala India 6 Department of Pediatric Hematology, Oncology, Blood and Marrow Transplantation, Apollo Hospitals, 320, Padma complex, Anna salai, Teynampet, Chennai, Tamil Nadu, India 7 Department of Pediatric Immunology and Leukocyte Biology, ICMR-National Institute of Immunohaematology, KEM Hospital, Parel, Mumbai, Maharashtra, India. # Joint first authors $ Corresponding author Vinod Scaria: [email protected] Sridhar Sivasubbu: [email protected] Geeta Madathil Govindaraj: [email protected] NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

Exome Sequencing and Functional Validation in Zebrafish Identify
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector REPORT Exome Sequencing and Functional Validation in Zebrafish Identify GTDC2 Mutations as a Cause of Walker-Warburg Syndrome M. Chiara Manzini,1,2 Dimira E. Tambunan,1,2 R. Sean Hill,1,2 Tim W. Yu,1,2 Thomas M. Maynard,3 Erin L. Heinzen,4 Kevin V. Shianna,4 Christine R. Stevens,5 Jennifer N. Partlow,1,2 Brenda J. Barry,1,2 Jacqueline Rodriguez,1,2 Vandana A. Gupta,1,6 Abdel-Karim Al-Qudah,7 Wafaa M. Eyaid,8 Jan M. Friedman,9,10 Mustafa A. Salih,11 Robin Clark,12 Isabella Moroni,13 Marina Mora,14 Alan H. Beggs,1,6 Stacey B. Gabriel,5 and Christopher A. Walsh1,2,5,* Whole-exome sequencing (WES), which analyzes the coding sequence of most annotated genes in the human genome, is an ideal approach to studying fully penetrant autosomal-recessive diseases, and it has been very powerful in identifying disease-causing muta- tions even when enrollment of affected individuals is limited by reduced survival. In this study, we combined WES with homozygosity analysis of consanguineous pedigrees, which are informative even when a single affected individual is available, to identify genetic mutations responsible for Walker-Warburg syndrome (WWS), a genetically heterogeneous autosomal-recessive disorder that severely affects the development of the brain, eyes, and muscle. Mutations in seven genes are known to cause WWS and explain 50%–60% of cases, but multiple additional genes are expected to be mutated because unexplained cases show suggestive linkage to diverse loci. -

Genomic Approaches for Understanding the Genetics of Complex Disease
Downloaded from genome.cshlp.org on September 25, 2021 - Published by Cold Spring Harbor Laboratory Press Perspective Genomic approaches for understanding the genetics of complex disease William L. Lowe Jr.1 and Timothy E. Reddy2,3 1Division of Endocrinology, Metabolism and Molecular Medicine, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois 60611, USA; 2Department of Biostatistics and Bioinformatics, Duke University Medical School, Durham, North Carolina 27708, USA; 3Center for Genomic and Computational Biology, Duke University Medical School, Durham, North Carolina 27708, USA There are thousands of known associations between genetic variants and complex human phenotypes, and the rate of novel discoveries is rapidly increasing. Translating those associations into knowledge of disease mechanisms remains a fundamental challenge because the associated variants are overwhelmingly in noncoding regions of the genome where we have few guiding principles to predict their function. Intersecting the compendium of identified genetic associations with maps of regulatory activity across the human genome has revealed that phenotype-associated variants are highly enriched in candidate regula- tory elements. Allele-specific analyses of gene regulation can further prioritize variants that likely have a functional effect on disease mechanisms; and emerging high-throughput assays to quantify the activity of candidate regulatory elements are a promising next step in that direction. Together, these technologies have created the ability to systematically and empirically test hypotheses about the function of noncoding variants and haplotypes at the scale needed for comprehensive and system- atic follow-up of genetic association studies. Major coordinated efforts to quantify regulatory mechanisms across genetically diverse populations in increasingly realistic cell models would be highly beneficial to realize that potential. -

A Comprehensive Database of Putative Human Microrna Target Site
bioRxiv preprint doi: https://doi.org/10.1101/554485; this version posted May 26, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. dbMTS: a comprehensive database of putative human microRNA target site SNVs and their functional predictions Chang Li1,2, Michael D. Swartz3, Bing Yu1, Yongsheng Bai4, Xiaoming Liu1,2* 1Human Genetics Center and Department of Epidemiology, Human Genetics and Environmental Sciences, School of Public Health, The University of Texas Health Science Center at Houston, Houston, TX 2USF Genomics, College of Public Health, University of South Florida, Tampa, FL 3Department of Biostatistics, School of Public Health, The University of Texas Health Science Center at Houston, Houston, TX 4Department of Internal Medicine, University of Michigan, Ann Arbor, MI *Correspondence: Xiaoming Liu, [email protected] Funding information: This work is supported partially by NIH funding 1UM1HG008898 and partially by the startup funding for XL from the University of South Florida. bioRxiv preprint doi: https://doi.org/10.1101/554485; this version posted May 26, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract microRNAs (miRNAs) are short non-coding RNAs that can repress the expression of protein coding messenger RNAs (mRNAs) by binding to the 3’UTR of the target. -

Interleukin 10 Mutant Zebrafish Have an Enhanced Interferon
www.nature.com/scientificreports OPEN Interleukin 10 mutant zebrafsh have an enhanced interferon gamma response and improved Received: 22 August 2017 Accepted: 20 June 2018 survival against a Mycobacterium Published: xx xx xxxx marinum infection Sanna-Kaisa E. Harjula1, Markus J. T. Ojanen1,2, Sinja Taavitsainen3, Matti Nykter3 & Mika Rämet1,4,5,6 Tuberculosis ranks as one of the world’s deadliest infectious diseases causing more than a million casualties annually. IL10 inhibits the function of Th1 type cells, and IL10 defciency has been associated with an improved resistance against Mycobacterium tuberculosis infection in a mouse model. Here, we utilized M. marinum infection in the zebrafsh (Danio rerio) as a model for studying Il10 in the host response against mycobacteria. Unchallenged, nonsense il10e46/e46 mutant zebrafsh were fertile and phenotypically normal. Following a chronic mycobacterial infection, il10e46/e46 mutants showed enhanced survival compared to the controls. This was associated with an increased expression of the Th cell marker cd4-1 and a shift towards a Th1 type immune response, which was demonstrated by the upregulated expression of tbx21 and ifng1, as well as the down-regulation of gata3. In addition, at 8 weeks post infection il10e46/e46 mutant zebrafsh had reduced expression levels of proinfammatory cytokines tnf and il1b, presumably indicating slower progress of the infection. Altogether, our data show that Il10 can weaken the immune defense against M. marinum infection in zebrafsh by restricting ifng1 response. Importantly, our fndings support the relevance of M. marinum infection in zebrafsh as a model for tuberculosis. Annually more than 10 million new tuberculosis cases are estimated to emerge, leading to over a million casualties1. -

Comparison of Structural and Short Variants Detectedby Linked-Read
cancers Article Comparison of Structural and Short Variants Detected by Linked-Read and Whole-Exome Sequencing in Multiple Myeloma Ashwini Kumar 1,2 , Sadiksha Adhikari 1,2, Matti Kankainen 2,3,4,5 and Caroline A. Heckman 1,2,* 1 Institute for Molecular Medicine Finland-FIMM, HiLIFE-Helsinki Institute of Life Science, iCAN Digital Cancer Medicine Flagship, University of Helsinki, Tukholmankatu 8, 00290 Helsinki, Finland; ashwini.kumar@helsinki.fi (A.K.); sadiksha.adhikari@helsinki.fi (S.A.) 2 iCAN Digital Precision Cancer Medicine, University of Helsinki, 00014 Helsinki, Finland; matti.kankainen@helsinki.fi 3 Medical and Clinical Genetics, University of Helsinki, Helsinki University Hospital, 00029 Helsinki, Finland 4 Translational Immunology Research Program and Department of Clinical Chemistry, University of Helsinki, 00290 Helsinki, Finland 5 Hematology Research Unit Helsinki, Department of Hematology, Helsinki University Hospital Comprehensive Cancer Center, 00290 Helsinki, Finland * Correspondence: caroline.heckman@helsinki.fi; Tel.: +358-29-412-5769 Simple Summary: The wide variety of next-generation sequencing technologies requires thorough evaluation and understanding of their advantages and shortcomings of these different approaches prior to their implementation in a precision medicine setting. Here, we compared the performance of two DNA sequencing methods, whole-exome and linked-read exome sequencing, to detect large Citation: Kumar, A.; Adhikari, S.; structural variants (SVs) and short variants in eight multiple myeloma (MM) patient cases. For three Kankainen, M.; Heckman, C.A. patient cases, matched tumor-normal samples were sequenced with both methods to compare somatic Comparison of Structural and Short SVs and short variants. The methods’ clinical relevance was also evaluated, and their sensitivity Variants Detected by Linked-Read and specificity to detect MM-specific cytogenetic alterations and other short variants were measured. -

Variant Annotation
Variant annotation Theorical part V1.1 10/10/18 Ready for annotating variants!! Variant annotation and prioritization Annotation Databases Eilbeck, Karen & Quinlan, Aaron & Yandell, Mark. (2017). Settling the score: variant prioritization and Mendelian disease. Nature Reviews Genetics. 10.1038/nrg.2017.52. Genomic data repositories - 1000 Genomes The 1000 Genomes Project (abbreviated as 1KGP), launched in January 2008, was an international research effort to establish by far the most detailed catalogue of human genetic variation. - ESP (NHLBI Exome Sequencing Project) Exists in 3 flavours : evs annotation data was generated from approximately 2500 exomes, evs_5400 from approximately 5400 exomes and the last one, evs_6500 from approximately 6500 exomes - ExAC (Exome Aggregation Consortium) Coalition of investigators seeking to aggregate and harmonize exome sequencing data from a variety of large-scale sequencing projects, and to make summary data available for the wider scientific community. - gnomAD (Genome Aggregation Database) Developed by an international coalition of investigators, with the goal of aggregating and harmonizing both exome and genome sequencing data from a wide variety of large-scale sequencing projects. - FREX (THE FRENCH EXOME) A REFERENCE PANEL OF EXOMES FROM FRENCH REGIONS Annotation Databases Eilbeck, Karen & Quinlan, Aaron & Yandell, Mark. (2017). Settling the score: variant prioritization and Mendelian disease. Nature Reviews Genetics. 10.1038/nrg.2017.52. Databases of variant-disease and gene-disease associations -

1 Mapping Challenging Mutations by Whole-Genome Sequencing Harold
bioRxiv preprint doi: https://doi.org/10.1101/036046; this version posted January 6, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Mapping challenging mutations by whole-genome sequencing Harold E. Smith, Amy S. Fabritius, Aimee Jaramillo-Lambert, and Andy Golden National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland 20892 1 bioRxiv preprint doi: https://doi.org/10.1101/036046; this version posted January 6, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Running title: Challenging mutations by WGS Keywords: Forward genetics, variant detection, complex alleles, SNP mapping Corresponding author: Harold E. Smith 8 Center Drive Bethesda, MD 20892 301-594-6554 [email protected] 2 bioRxiv preprint doi: https://doi.org/10.1101/036046; this version posted January 6, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. ABSTRACT Whole-genome sequencing provides a rapid and powerful method for identifying mutations on a global scale, and has spurred a renewed enthusiasm for classical genetic screens in model organisms. -
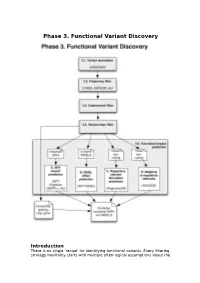
Phase 3. Functional Variant Discovery
Phase 3. Functional Variant Discovery Introduction There is no single ‘recipe’ for identifying functional variants. Every filtering strategy inevitably starts with multiple often-logical assumptions about the properties of causal variants that may also discard candidates too early. Novelty is one example, where the variant is not expected to be present in public repositories, even though apparently healthy individuals carrying it may have been sequenced in unrelated studies. High predicted functional impact is another, where a variant is expected to simultaneously occur at an evolutionarily conserved genomic location and also be scored as deleterious by multiple predictive algorithms may even be too stringent a rule in a Mendelian trait. In a similar vein, in a population genetics study, a novel amino acid class-changing variant that is not predicted to have a functional effect by the standard tools may have a molecular effect. An inheritance model may itself be incorrect, e.g. a condition may be autosomal recessive rather than X-linked as hypothesized, or digenic rather than recessive, etc. It is therefore important not to conclude too early that the variant(s) of interest “are not in the exome” and to exhaust all alternate plausible rules and scenarios before that conclusion is reached. Using disease variant discovery as a context, we aim to present here a range of guidelines that can be used to develop an appropriate variant prioritisation strategy. This phase will be presented as 5 sub-phases: 1. Variant annotation - using ANNOVAR (Wang et al, 2010) as an example 2. Frequency 3. Case-control filtering 4. Variant class filtering 5. -

A Pipeline for Identifying Saccharomyces Cerevisiae Mutations
SOFTWARE AND DATA RESOURCES MutantHuntWGS: A Pipeline for Identifying Saccharomyces cerevisiae Mutations Mitchell A. Ellison,1 Jennifer L. Walker, Patrick J. Ropp, Jacob D. Durrant, and Karen M. Arndt1 Department of Biological Sciences, University of Pittsburgh, PA, 15260 ORCID IDs: 0000-0002-5682-096X (M.A.E.); 0000-0003-1320-9957 (K.M.A.) ABSTRACT MutantHuntWGS is a user-friendly pipeline for analyzing Saccharomyces cerevisiae whole- KEYWORDS genome sequencing data. It uses available open-source programs to: (1) perform sequence alignments for Mutant hunt paired and single-end reads, (2) call variants, and (3) predict variant effect and severity. MutantHuntWGS variant calling outputs a shortlist of variants while also enabling access to all intermediate files. To demonstrate its utility, we Saccharomyces use MutantHuntWGS to assess multiple published datasets; in all cases, it detects the same causal variants cerevisiae reported in the literature. To encourage broad adoption and promote reproducibility, we distribute a bulk segregant containerized version of the MutantHuntWGS pipeline that allows users to install and analyze data with only analysis two commands. The MutantHuntWGS software and documentation can be downloaded free of charge from lab evolution https://github.com/mae92/MutantHuntWGS. Saccharomyces cerevisiae is a powerful model system for under- WGS is particularly powerful when used in conjunction with lab- standing the complex processes that direct cellular function and evolution (Goldgof et al. 2016; Ottilie et al. 2017) or mutant-hunt underpin many human diseases (Birkeland et al. 2010; Botstein experiments, both with (Birkeland et al. 2010; Reavey et al. 2015) and Fink 2011; Kachroo et al. 2015; Hamza et al. -

Gain-Of-Function Mutation of Microrna-140 in Human Skeletal Dysplasia
LETTERS https://doi.org/10.1038/s41591-019-0353-2 Gain-of-function mutation of microRNA-140 in human skeletal dysplasia Giedre Grigelioniene1,2,3,15, Hiroshi I. Suzuki 4,15, Fulya Taylan 2, Fatemeh Mirzamohammadi1, Zvi U. Borochowitz5, Ugur M. Ayturk6, Shay Tzur7,8, Eva Horemuzova9,10, Anna Lindstrand2,3, Mary Ann Weis11, Gintautas Grigelionis 2, Anna Hammarsjö 2,3, Elin Marsk12, Ann Nordgren2,3, Magnus Nordenskjöld2,3, David R. Eyre11, Matthew L. Warman 6, Gen Nishimura13, Phillip A. Sharp 4,14 and Tatsuya Kobayashi 1* MicroRNAs (miRNAs) are post-transcriptional regulators of A novel skeletal dysplasia was identified in two unrelated fam- gene expression. Heterozygous loss-of-function point muta- ilies in a project for molecular diagnosis of ultrarare congenital tions of miRNA genes are associated with several human con- skeletal disorders (Fig. 1a,b). In family 1, individual II-2, patient genital disorders1–5, but neomorphic (gain-of-new-function) 1, P1, is affected, as is one of her sons (III-2, P2). In family 2, II-1, mutations in miRNAs due to nucleotide substitutions have not P3 is the only affected individual. Clinical features of the skel- been reported. Here we describe a neomorphic seed region etal dysplasia included disproportionate short stature with short mutation in the chondrocyte-specific, super-enhancer-asso- limbs, small hands and feet, and midface hypoplasia with small ciated MIR140 gene encoding microRNA-140 (miR-140) in nose. The radiological hallmarks were mild spondylar dysplasia, a novel autosomal dominant human skeletal dysplasia. Mice delayed epiphyseal ossification of the hip and knee, and severe with the corresponding single nucleotide substitution show brachydactyly with cone-shaped phalangeal epiphyses (Fig. -
Improving Isobutanol Productivity Through Adaptive Laboratory Evolution in Saccharomyces Cerevisiae
Improving isobutanol productivity through adaptive laboratory evolution in Saccharomyces cerevisiae Aili Zhang ( [email protected] ) Hebei University of Technology Yide Su Hebei University of Techbology Jingzhi Li Hebei University of Technology Weiwei Zhang Hebei University of Technology Research Keywords: Saccharomyces cerevisiae, isobutanol, adaptive laboratory evolution, productivity Posted Date: December 20th, 2019 DOI: https://doi.org/10.21203/rs.2.19485/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/28 Abstract Background: Isobutanol is an ideal second-generation biofuels due to its lower hygroscopicity, higher energy density and higher-octane value. However, isobutanol is toxic to production organisms. To improve isobutanol productivity, adaptive laboratory evolution method was carried out to improve the tolerance of Saccharomyces cerevisiae toward higher isobutanol and higher glucose concentration. Results: We evolved the laboratory strain of S. cerevisiae W303-1A by using EMS (ethyl methanesulfonate) mutagenesis followed by adaptive laboratory evolution. The evolved strain EMS39 with signicant increase in growth rate and viability in media with higher isobutanol and higher glucose concentration was obtained. Then, metabolic engineering of the evolved strain EMS39 as a platform for isobutanol production were carried out. Delta integration method was used to over-express ILV3 gene and 2μ plasmids carrying ILV2, ILV5 and ARO10 were used to over-express ILV2, ILV5 and ARO10 genes in the evolved strain EMS39 and wild type W303-1A. And the resulting strains was designated as strain EMS39V2δV3V5A10 and strain W303-1AV2δV3V5A10, respectively. Our results shown that isobutanol titers of the evolved strain EMS39 increased by 30% compared to the control strain.