A Wave Packet in a Square Well (An Electron in a Box) Changing with Time. Last Time: Light
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Accomplishments in Nanotechnology
U.S. Department of Commerce Carlos M. Gutierrez, Secretaiy Technology Administration Robert Cresanti, Under Secretaiy of Commerce for Technology National Institute ofStandards and Technolog}' William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment used are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publication 1052 Natl. Inst. Stand. Technol. Spec. Publ. 1052, 186 pages (August 2006) CODEN: NSPUE2 NIST Special Publication 1052 Accomplishments in Nanoteciinology Compiled and Edited by: Michael T. Postek, Assistant to the Director for Nanotechnology, Manufacturing Engineering Laboratory Joseph Kopanski, Program Office and David Wollman, Electronics and Electrical Engineering Laboratory U. S. Department of Commerce Technology Administration National Institute of Standards and Technology Gaithersburg, MD 20899 August 2006 National Institute of Standards and Teclinology • Technology Administration • U.S. Department of Commerce Acknowledgments Thanks go to the NIST technical staff for providing the information outlined on this report. Each of the investigators is identified with their contribution. Contact information can be obtained by going to: http ://www. nist.gov Acknowledged as well, -

Unit 1 Old Quantum Theory
UNIT 1 OLD QUANTUM THEORY Structure Introduction Objectives li;,:overy of Sub-atomic Particles Earlier Atom Models Light as clectromagnetic Wave Failures of Classical Physics Black Body Radiation '1 Heat Capacity Variation Photoelectric Effect Atomic Spectra Planck's Quantum Theory, Black Body ~diation. and Heat Capacity Variation Einstein's Theory of Photoelectric Effect Bohr Atom Model Calculation of Radius of Orbits Energy of an Electron in an Orbit Atomic Spectra and Bohr's Theory Critical Analysis of Bohr's Theory Refinements in the Atomic Spectra The61-y Summary Terminal Questions Answers 1.1 INTRODUCTION The ideas of classical mechanics developed by Galileo, Kepler and Newton, when applied to atomic and molecular systems were found to be inadequate. Need was felt for a theory to describe, correlate and predict the behaviour of the sub-atomic particles. The quantum theory, proposed by Max Planck and applied by Einstein and Bohr to explain different aspects of behaviour of matter, is an important milestone in the formulation of the modern concept of atom. In this unit, we will study how black body radiation, heat capacity variation, photoelectric effect and atomic spectra of hydrogen can be explained on the basis of theories proposed by Max Planck, Einstein and Bohr. They based their theories on the postulate that all interactions between matter and radiation occur in terms of definite packets of energy, known as quanta. Their ideas, when extended further, led to the evolution of wave mechanics, which shows the dual nature of matter -

Federico Capasso “Physics by Design: Engineering Our Way out of the Thz Gap” Peter H
6 IEEE TRANSACTIONS ON TERAHERTZ SCIENCE AND TECHNOLOGY, VOL. 3, NO. 1, JANUARY 2013 Terahertz Pioneer: Federico Capasso “Physics by Design: Engineering Our Way Out of the THz Gap” Peter H. Siegel, Fellow, IEEE EDERICO CAPASSO1credits his father, an economist F and business man, for nourishing his early interest in science, and his mother for making sure he stuck it out, despite some tough moments. However, he confesses his real attraction to science came from a well read children’s book—Our Friend the Atom [1], which he received at the age of 7, and recalls fondly to this day. I read it myself, but it did not do me nearly as much good as it seems to have done for Federico! Capasso grew up in Rome, Italy, and appropriately studied Latin and Greek in his pre-university days. He recalls that his father wisely insisted that he and his sister become fluent in English at an early age, noting that this would be a more im- portant opportunity builder in later years. In the 1950s and early 1960s, Capasso remembers that for his family of friends at least, physics was the king of sciences in Italy. There was a strong push into nuclear energy, and Italy had a revered first son in En- rico Fermi. When Capasso enrolled at University of Rome in FREDERICO CAPASSO 1969, it was with the intent of becoming a nuclear physicist. The first two years were extremely difficult. University of exams, lack of grade inflation and rigorous course load, had Rome had very high standards—there were at least three faculty Capasso rethinking his career choice after two years. -

A Study of Fractional Schrödinger Equation-Composed Via Jumarie Fractional Derivative
A Study of Fractional Schrödinger Equation-composed via Jumarie fractional derivative Joydip Banerjee1, Uttam Ghosh2a , Susmita Sarkar2b and Shantanu Das3 Uttar Buincha Kajal Hari Primary school, Fulia, Nadia, West Bengal, India email- [email protected] 2Department of Applied Mathematics, University of Calcutta, Kolkata, India; 2aemail : [email protected] 2b email : [email protected] 3 Reactor Control Division BARC Mumbai India email : [email protected] Abstract One of the motivations for using fractional calculus in physical systems is due to fact that many times, in the space and time variables we are dealing which exhibit coarse-grained phenomena, meaning that infinitesimal quantities cannot be placed arbitrarily to zero-rather they are non-zero with a minimum length. Especially when we are dealing in microscopic to mesoscopic level of systems. Meaning if we denote x the point in space andt as point in time; then the differentials dx (and dt ) cannot be taken to limit zero, rather it has spread. A way to take this into account is to use infinitesimal quantities as ()Δx α (and ()Δt α ) with 01<α <, which for very-very small Δx (and Δt ); that is trending towards zero, these ‘fractional’ differentials are greater that Δx (and Δt ). That is()Δx α >Δx . This way defining the differentials-or rather fractional differentials makes us to use fractional derivatives in the study of dynamic systems. In fractional calculus the fractional order trigonometric functions play important role. The Mittag-Leffler function which plays important role in the field of fractional calculus; and the fractional order trigonometric functions are defined using this Mittag-Leffler function. -

Quantum Theory of the Hydrogen Atom
Quantum Theory of the Hydrogen Atom Chemistry 35 Fall 2000 Balmer and the Hydrogen Spectrum n 1885: Johann Balmer, a Swiss schoolteacher, empirically deduced a formula which predicted the wavelengths of emission for Hydrogen: l (in Å) = 3645.6 x n2 for n = 3, 4, 5, 6 n2 -4 •Predicts the wavelengths of the 4 visible emission lines from Hydrogen (which are called the Balmer Series) •Implies that there is some underlying order in the atom that results in this deceptively simple equation. 2 1 The Bohr Atom n 1913: Niels Bohr uses quantum theory to explain the origin of the line spectrum of hydrogen 1. The electron in a hydrogen atom can exist only in discrete orbits 2. The orbits are circular paths about the nucleus at varying radii 3. Each orbit corresponds to a particular energy 4. Orbit energies increase with increasing radii 5. The lowest energy orbit is called the ground state 6. After absorbing energy, the e- jumps to a higher energy orbit (an excited state) 7. When the e- drops down to a lower energy orbit, the energy lost can be given off as a quantum of light 8. The energy of the photon emitted is equal to the difference in energies of the two orbits involved 3 Mohr Bohr n Mathematically, Bohr equated the two forces acting on the orbiting electron: coulombic attraction = centrifugal accelleration 2 2 2 -(Z/4peo)(e /r ) = m(v /r) n Rearranging and making the wild assumption: mvr = n(h/2p) n e- angular momentum can only have certain quantified values in whole multiples of h/2p 4 2 Hydrogen Energy Levels n Based on this model, Bohr arrived at a simple equation to calculate the electron energy levels in hydrogen: 2 En = -RH(1/n ) for n = 1, 2, 3, 4, . -

Lecture 16 Matter Waves & Wave Functions
LECTURE 16 MATTER WAVES & WAVE FUNCTIONS Instructor: Kazumi Tolich Lecture 16 2 ¨ Reading chapter 34-5 to 34-8 & 34-10 ¤ Electrons and matter waves n The de Broglie hypothesis n Electron interference and diffraction ¤ Wave-particle duality ¤ Standing waves and energy quantization ¤ Wave function ¤ Uncertainty principle ¤ A particle in a box n Standing wave functions de Broglie hypothesis 3 ¨ In 1924 Louis de Broglie hypothesized: ¤ Since light exhibits particle-like properties and act as a photon, particles could exhibit wave-like properties and have a definite wavelength. ¨ The wavelength and frequency of matter: # & � = , and � = $ # ¤ For macroscopic objects, de Broglie wavelength is too small to be observed. Example 1 4 ¨ One of the smallest composite microscopic particles we could imagine using in an experiment would be a particle of smoke or soot. These are about 1 µm in diameter, barely at the resolution limit of most microscopes. A particle of this size with the density of carbon has a mass of about 10-18 kg. What is the de Broglie wavelength for such a particle, if it is moving slowly at 1 mm/s? Diffraction of matter 5 ¨ In 1927, C. J. Davisson and L. H. Germer first observed the diffraction of electron waves using electrons scattered from a particular nickel crystal. ¨ G. P. Thomson (son of J. J. Thomson who discovered electrons) showed electron diffraction when the electrons pass through a thin metal foils. ¨ Diffraction has been seen for neutrons, hydrogen atoms, alpha particles, and complicated molecules. ¨ In all cases, the measured λ matched de Broglie’s prediction. X-ray diffraction electron diffraction neutron diffraction Interference of matter 6 ¨ If the wavelengths are made long enough (by using very slow moving particles), interference patters of particles can be observed. -

Vibrational Quantum Number
Fundamentals in Biophotonics Quantum nature of atoms, molecules – matter Aleksandra Radenovic [email protected] EPFL – Ecole Polytechnique Federale de Lausanne Bioengineering Institute IBI 26. 03. 2018. Quantum numbers •The four quantum numbers-are discrete sets of integers or half- integers. –n: Principal quantum number-The first describes the electron shell, or energy level, of an atom –ℓ : Orbital angular momentum quantum number-as the angular quantum number or orbital quantum number) describes the subshell, and gives the magnitude of the orbital angular momentum through the relation Ll2 ( 1) –mℓ:Magnetic (azimuthal) quantum number (refers, to the direction of the angular momentum vector. The magnetic quantum number m does not affect the electron's energy, but it does affect the probability cloud)- magnetic quantum number determines the energy shift of an atomic orbital due to an external magnetic field-Zeeman effect -s spin- intrinsic angular momentum Spin "up" and "down" allows two electrons for each set of spatial quantum numbers. The restrictions for the quantum numbers: – n = 1, 2, 3, 4, . – ℓ = 0, 1, 2, 3, . , n − 1 – mℓ = − ℓ, − ℓ + 1, . , 0, 1, . , ℓ − 1, ℓ – –Equivalently: n > 0 The energy levels are: ℓ < n |m | ≤ ℓ ℓ E E 0 n n2 Stern-Gerlach experiment If the particles were classical spinning objects, one would expect the distribution of their spin angular momentum vectors to be random and continuous. Each particle would be deflected by a different amount, producing some density distribution on the detector screen. Instead, the particles passing through the Stern–Gerlach apparatus are deflected either up or down by a specific amount. -

The Quantum Mechanical Model of the Atom
The Quantum Mechanical Model of the Atom Quantum Numbers In order to describe the probable location of electrons, they are assigned four numbers called quantum numbers. The quantum numbers of an electron are kind of like the electron’s “address”. No two electrons can be described by the exact same four quantum numbers. This is called The Pauli Exclusion Principle. • Principle quantum number: The principle quantum number describes which orbit the electron is in and therefore how much energy the electron has. - it is symbolized by the letter n. - positive whole numbers are assigned (not including 0): n=1, n=2, n=3 , etc - the higher the number, the further the orbit from the nucleus - the higher the number, the more energy the electron has (this is sort of like Bohr’s energy levels) - the orbits (energy levels) are also called shells • Angular momentum (azimuthal) quantum number: The azimuthal quantum number describes the sublevels (subshells) that occur in each of the levels (shells) described above. - it is symbolized by the letter l - positive whole number values including 0 are assigned: l = 0, l = 1, l = 2, etc. - each number represents the shape of a subshell: l = 0, represents an s subshell l = 1, represents a p subshell l = 2, represents a d subshell l = 3, represents an f subshell - the higher the number, the more complex the shape of the subshell. The picture below shows the shape of the s and p subshells: (notice the electron clouds) • Magnetic quantum number: All of the subshells described above (except s) have more than one orientation. -

4 Nuclear Magnetic Resonance
Chapter 4, page 1 4 Nuclear Magnetic Resonance Pieter Zeeman observed in 1896 the splitting of optical spectral lines in the field of an electromagnet. Since then, the splitting of energy levels proportional to an external magnetic field has been called the "Zeeman effect". The "Zeeman resonance effect" causes magnetic resonances which are classified under radio frequency spectroscopy (rf spectroscopy). In these resonances, the transitions between two branches of a single energy level split in an external magnetic field are measured in the megahertz and gigahertz range. In 1944, Jevgeni Konstantinovitch Savoiski discovered electron paramagnetic resonance. Shortly thereafter in 1945, nuclear magnetic resonance was demonstrated almost simultaneously in Boston by Edward Mills Purcell and in Stanford by Felix Bloch. Nuclear magnetic resonance was sometimes called nuclear induction or paramagnetic nuclear resonance. It is generally abbreviated to NMR. So as not to scare prospective patients in medicine, reference to the "nuclear" character of NMR is dropped and the magnetic resonance based imaging systems (scanner) found in hospitals are simply referred to as "magnetic resonance imaging" (MRI). 4.1 The Nuclear Resonance Effect Many atomic nuclei have spin, characterized by the nuclear spin quantum number I. The absolute value of the spin angular momentum is L =+h II(1). (4.01) The component in the direction of an applied field is Lz = Iz h ≡ m h. (4.02) The external field is usually defined along the z-direction. The magnetic quantum number is symbolized by Iz or m and can have 2I +1 values: Iz ≡ m = −I, −I+1, ..., I−1, I. -

Dirac Particle in a Square Well and in a Box
Dirac particle in a square well and in a box A. D. Alhaidari Saudi Center for Theoretical Physics, Dhahran, Saudi Arabia We obtain an exact solution of the 1D Dirac equation for a square well potential of depth greater then twice the particle’s mass. The energy spectrum formula in the Klein zone is surprisingly very simple and independent of the depth of the well. This implies that the same solution is also valid for the potential box (infinitely deep well). In the nonrelativistic limit, the well-known energy spectrum of a particle in a box is obtained. We also provide in tabular form the elements of the complete solution space of the problem for all energies. PACS numbers: 03.65.Pm, 03.65.Ge, 03.65.Nk Keywords: Dirac equation, square well, potential box, energy spectrum, Klein paradox Aside from the mathematically “trivial” free case, particle in a box is usually the first problem that an undergraduate student of nonrelativistic quantum mechanics is asked to solve. Normally, he goes further into obtaining the bound states solution of a particle in a finite square well. It is much later that he works out more involved exercises like the 3D Coulomb problem [1]. Unfortunately, in relativistic quantum mechanics the story is reversed. One can hardly find a textbook on relativistic quantum mechanics where the 1D problem of a particle in a potential box is solved before the relativistic Hydrogen atom is [2]. The difficulty is that if this is to be done from the start, then one is forced to get into subtle issues like the Klein paradox, electron-positron pair production, stability of the vacuum, appropriate boundary conditions, …etc. -

A Relativistic Electron in a Coulomb Potential
A Relativistic Electron in a Coulomb Potential Alfred Whitehead Physics 518, Fall 2009 The Problem Solve the Dirac Equation for an electron in a Coulomb potential. Identify the conserved quantum numbers. Specify the degeneracies. Compare with solutions of the Schrödinger equation including relativistic and spin corrections. Approach My approach follows that taken by Dirac in [1] closely. A few modifications taken from [2] and [3] are included, particularly in regards to the final quantum numbers chosen. The general strategy is to first find a set of transformations which turn the Hamiltonian for the system into a form that depends only on the radial variables r and pr. Once this form is found, I solve it to find the energy eigenvalues and then discuss the energy spectrum. The Radial Dirac Equation We begin with the electromagnetic Hamiltonian q H = p − cρ ~σ · ~p − A~ + ρ mc2 (1) 0 1 c 3 with 2 0 0 1 0 3 6 0 0 0 1 7 ρ1 = 6 7 (2) 4 1 0 0 0 5 0 1 0 0 2 1 0 0 0 3 6 0 1 0 0 7 ρ3 = 6 7 (3) 4 0 0 −1 0 5 0 0 0 −1 1 2 0 1 0 0 3 2 0 −i 0 0 3 2 1 0 0 0 3 6 1 0 0 0 7 6 i 0 0 0 7 6 0 −1 0 0 7 ~σ = 6 7 ; 6 7 ; 6 7 (4) 4 0 0 0 1 5 4 0 0 0 −i 5 4 0 0 1 0 5 0 0 1 0 0 0 i 0 0 0 0 −1 We note that, for the Coulomb potential, we can set (using cgs units): Ze2 p = −eΦ = − o r A~ = 0 This leads us to this form for the Hamiltonian: −Ze2 H = − − cρ ~σ · ~p + ρ mc2 (5) r 1 3 We need to get equation 5 into a form which depends not on ~p, but only on the radial variables r and pr. -
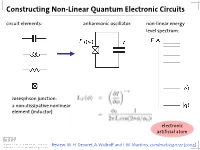
Constructing Non-Linear Quantum Electronic Circuits Circuit Elements: Anharmonic Oscillator: Non-Linear Energy Level Spectrum
Constructing Non-Linear Quantum Electronic Circuits circuit elements: anharmonic oscillator: non-linear energy level spectrum: Josesphson junction: a non-dissipative nonlinear element (inductor) electronic artificial atom Review: M. H. Devoret, A. Wallraff and J. M. Martinis, condmat/0411172 (2004) A Classification of Josephson Junction Based Qubits How to make use in of Jospehson junctions in a qubit? Common options of bias (control) circuits: phase qubit charge qubit flux qubit (Cooper Pair Box, Transmon) current bias charge bias flux bias How is the control circuit important? The Cooper Pair Box Qubit A Charge Qubit: The Cooper Pair Box discrete charge on island: continuous gate charge: total box capacitance Hamiltonian: electrostatic part: charging energy magnetic part: Josephson energy Hamilton Operator of the Cooper Pair Box Hamiltonian: commutation relation: charge number operator: eigenvalues, eigenfunctions completeness orthogonality phase basis: basis transformation Solving the Cooper Pair Box Hamiltonian Hamilton operator in the charge basis N : solutions in the charge basis: Hamilton operator in the phase basis δ : transformation of the number operator: solutions in the phase basis: Energy Levels energy level diagram for EJ=0: • energy bands are formed • bands are periodic in Ng energy bands for finite EJ • Josephson coupling lifts degeneracy • EJ scales level separation at charge degeneracy Charge and Phase Wave Functions (EJ << EC) courtesy CEA Saclay Charge and Phase Wave Functions (EJ ~ EC) courtesy CEA Saclay Tuning the Josephson Energy split Cooper pair box in perpendicular field = ( ) , cos cos 2 � � − − ̂ 0 SQUID modulation of Josephson energy consider two state approximation J. Clarke, Proc. IEEE 77, 1208 (1989) Two-State Approximation Restricting to a two-charge Hilbert space: Shnirman et al., Phys.