Chapter 2: Interactions of Radiation with Matter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Measurements of Elastic Electron-Proton Scattering at Large Momentum Transfer*
SLAC-PUB-4395 Rev. January 1993 m Measurements of Elastic Electron-Proton Scattering at Large Momentum Transfer* A. F. SILL,(~) R. G. ARNOLD,P. E. BOSTED,~. C. CHANG,@) J. GoMEz,(~)A. T. KATRAMATOU,C. J. MARTOFF, G. G. PETRATOS,(~)A. A. RAHBAR,S. E. ROCK,AND Z. M. SZALATA Department of Physics The American University, Washington DC 20016 D.J. SHERDEN Stanford Linear Accelerator Center , Stanford University, Stanford, California 94309 J. M. LAMBERT Department of Physics Georgetown University, Washington DC 20057 and R. M. LOMBARD-NELSEN De’partemente de Physique Nucle’aire CEN Saclay, Gif-sur- Yvette, 91191 Cedex, France Submitted to Physical Review D *Work supported by US Department of Energy contract DE-AC03-76SF00515 (SLAC), and National Science Foundation Grants PHY83-40337 and PHY85-10549 (American University). R. M. Lombard-Nelsen was supported by C. N. R. S. (French National Center for Scientific Research). Javier Gomez was partially supported by CONICIT, Venezuela. (‘)Present address: Department of Physics and Astronomy, University of Rochester, NY 14627. (*)Permanent address: Department of Physics and Astronomy, University of Maryland, College Park, MD 20742. (C)Present address: Continuous Electron Beam Accelerator Facility, Newport News, VA 23606. (d)Present address: Temple University, Philadelphia, PA 19122. (c)Present address: Stanford Linear Accelerator Center, Stanford CA 94309. ABSTRACT Measurements of the forward-angle differential cross section for elastic electron-proton scattering were made in the range of momentum transfer from Q2 = 2.9 to 31.3 (GeV/c)2 using an electron beam at the Stanford Linear Accel- erator Center. The data span six orders of magnitude in cross section. -

Lecture 6: Spectroscopy and Photochemistry II
Lecture 6: Spectroscopy and Photochemistry II Required Reading: FP Chapter 3 Suggested Reading: SP Chapter 3 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring 2005 Prof. Jose-Luis Jimenez Outline of Lecture • The Sun as a radiation source • Attenuation from the atmosphere – Scattering by gases & aerosols – Absorption by gases • Beer-Lamber law • Atmospheric photochemistry – Calculation of photolysis rates – Radiation fluxes – Radiation models 1 Reminder of EM Spectrum Blackbody Radiation Linear Scale Log Scale From R.P. Turco, Earth Under Siege: From Air Pollution to Global Change, Oxford UP, 2002. 2 Solar & Earth Radiation Spectra • Sun is a radiation source with an effective blackbody temperature of about 5800 K • Earth receives circa 1368 W/m2 of energy from solar radiation From Turco From S. Nidkorodov • Question: are relative vertical scales ok in right plot? Solar Radiation Spectrum II From F-P&P •Solar spectrum is strongly modulated by atmospheric scattering and absorption From Turco 3 Solar Radiation Spectrum III UV Photon Energy ↑ C B A From Turco Solar Radiation Spectrum IV • Solar spectrum is strongly O3 modulated by atmospheric absorptions O 2 • Remember that UV photons have most energy –O2 absorbs extreme UV in mesosphere; O3 absorbs most UV in stratosphere – Chemistry of those regions partially driven by those absorptions – Only light with λ>290 nm penetrates into the lower troposphere – Biomolecules have same bonds (e.g. C-H), bonds can break with UV absorption => damage to life • Importance of protection From F-P&P provided by O3 layer 4 Solar Radiation Spectrum vs. altitude From F-P&P • Very high energy photons are depleted high up in the atmosphere • Some photochemistry is possible in stratosphere but not in troposphere • Only λ > 290 nm in trop. -

7. Gamma and X-Ray Interactions in Matter
Photon interactions in matter Gamma- and X-Ray • Compton effect • Photoelectric effect Interactions in Matter • Pair production • Rayleigh (coherent) scattering Chapter 7 • Photonuclear interactions F.A. Attix, Introduction to Radiological Kinematics Physics and Radiation Dosimetry Interaction cross sections Energy-transfer cross sections Mass attenuation coefficients 1 2 Compton interaction A.H. Compton • Inelastic photon scattering by an electron • Arthur Holly Compton (September 10, 1892 – March 15, 1962) • Main assumption: the electron struck by the • Received Nobel prize in physics 1927 for incoming photon is unbound and stationary his discovery of the Compton effect – The largest contribution from binding is under • Was a key figure in the Manhattan Project, condition of high Z, low energy and creation of first nuclear reactor, which went critical in December 1942 – Under these conditions photoelectric effect is dominant Born and buried in • Consider two aspects: kinematics and cross Wooster, OH http://en.wikipedia.org/wiki/Arthur_Compton sections http://www.findagrave.com/cgi-bin/fg.cgi?page=gr&GRid=22551 3 4 Compton interaction: Kinematics Compton interaction: Kinematics • An earlier theory of -ray scattering by Thomson, based on observations only at low energies, predicted that the scattered photon should always have the same energy as the incident one, regardless of h or • The failure of the Thomson theory to describe high-energy photon scattering necessitated the • Inelastic collision • After the collision the electron departs -

Neutron Scattering
Neutron Scattering Basic properties of neutron and electron neutron electron −27 −31 mass mkn =×1.675 10 g mke =×9.109 10 g charge 0 e spin s = ½ s = ½ −e= −e= magnetic dipole moment µnn= gs with gn = 3.826 µee= gs with ge = 2.0 2mn 2me =22k 2π =22k Ek== E = 2m λ 2m energy n e 81.81 150.26 Em[]eV = 2 Ee[]V = 2 λ ⎣⎡Å⎦⎤ λ ⎣⎦⎡⎤Å interaction with matter: Coulomb interaction — 9 strong-force interaction 9 — magnetic dipole-dipole 9 9 interaction Several salient features are apparent from this table: – electrons are charged and experience strong, long-range Coulomb interactions in a solid. They therefore typically only penetrate a few atomic layers into the solid. Electron scattering is therefore a surface-sensitive probe. Neutrons are uncharged and do not experience Coulomb interaction. The strong-force interaction is naturally strong but very short-range, and the magnetic interaction is long-range but weak. Neutrons therefore penetrate deeply into most materials, so that neutron scattering is a bulk probe. – Electrons with wavelengths comparable to interatomic distances (λ ~2Å ) have energies of several tens of electron volts, comparable to energies of plasmons and interband transitions in solids. Electron scattering is therefore well suited as a probe of these high-energy excitations. Neutrons with λ ~2Å have energies of several tens of meV , comparable to the thermal energies kTB at room temperature. These so-called “thermal neutrons” are excellent probes of low-energy excitations such as lattice vibrations and spin waves with energies in the meV range. -

12 Scattering in Three Dimensions
12 Scattering in three dimensions 12.1 Cross sections and geometry Most experiments in physics consist of sending one particle to collide with another, and looking at what comes out. The quantity we can usually measure is the scattering cross section: by analogy with classical scattering of hard spheres, we assuming that scattering occurs if the particles ‘hit’ each other. The cross section is the apparent ‘target area’. The total scattering cross section can be determined by the reduction in intensity of a beam of particles passing through a region on ‘targets’, while the differential scattering cross section requires detecting the scattered particles at different angles. We will use spherical polar coordinates, with the scattering potential located at the origin and the plane wave incident flux parallel to the z direction. In this coordinate system, scattering processes dσ are symmetric about φ, so dΩ will be independent of φ. We will also use a purely classical concept, the impact parameter b which is defined as the distance of the incident particle from the z-axis prior to scattering. S(k) δΩ I(k) θ z φ Figure 11: Standard spherical coordinate geometry for scattering 12.2 The Born Approximation We can use time-dependent perturbation theory to do an approximate calculation of the cross- section. Provided that the interaction between particle and scattering centre is localised to the region around r = 0, we can regard the incident and scattered particles as free when they are far from the scattering centre. We just need the result that we obtained for a constant perturbation, Fermi’s Golden Rule, to compute the rate of transitions between the initial state (free particle of momentum p) to the final state (free particle of momentum p0). -

Photon Cross Sections, Attenuation Coefficients, and Energy Absorption Coefficients from 10 Kev to 100 Gev*
1 of Stanaaros National Bureau Mmin. Bids- r'' Library. Ml gEP 2 5 1969 NSRDS-NBS 29 . A111D1 ^67174 tioton Cross Sections, i NBS Attenuation Coefficients, and & TECH RTC. 1 NATL INST OF STANDARDS _nergy Absorption Coefficients From 10 keV to 100 GeV U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS T X J ". j NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation’s central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services and the following technical divisions: Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic and Molec- ular Physics—Radio Physics -—Radio Engineering -—Time and Frequency -—Astro- physics -—Cryogenics. -

Calculation of Photon Attenuation Coefficients of Elements And
732 ISSN 0214-087X Calculation of photon attenuation coeffrcients of elements and compound Roteta, M.1 Baró, 2 Fernández-Varea, J.M.3 Salvat, F.3 1 CIEMAT. Avenida Complutense 22. 28040 Madrid, Spain. 2 Servéis Científico-Técnics, Universitat de Barcelona. Martí i Franqués s/n. 08028 Barcelona, Spain. 3 Facultat de Física (ECM), Universitat de Barcelona. Diagonal 647. 08028 Barcelona, Spain. CENTRO DE INVESTIGACIONES ENERGÉTICAS, MEDIOAMBIENTALES Y TECNOLÓGICAS MADRID, 1994 CLASIFICACIÓN DOE Y DESCRIPTORES: 990200 662300 COMPUTER LODES COMPUTER CALCULATIONS FORTRAN PROGRAMMING LANGUAGES CROSS SECTIONS PHOTONS Toda correspondencia en relación con este trabajo debe dirigirse al Servicio de Información y Documentación, Centro de Investigaciones Energéticas, Medioam- bientales y Tecnológicas, Ciudad Universitaria, 28040-MADRID, ESPAÑA. Las solicitudes de ejemplares deben dirigirse a este mismo Servicio. Los descriptores se han seleccionado del Thesauro del DOE para describir las materias que contiene este informe con vistas a su recuperación. La catalogación se ha hecho utilizando el documento DOE/TIC-4602 (Rev. 1) Descriptive Cataloguing On- Line, y la clasificación de acuerdo con el documento DOE/TIC.4584-R7 Subject Cate- gories and Scope publicados por el Office of Scientific and Technical Information del Departamento de Energía de los Estados Unidos. Se autoriza la reproducción de los resúmenes analíticos que aparecen en esta publicación. Este trabajo se ha recibido para su impresión en Abril de 1993 Depósito Legal n° M-14874-1994 ISBN 84-7834-235-4 ISSN 0214-087-X ÑIPO 238-94-013-4 IMPRIME CIEMAT Calculation of photon attenuation coefíicients of elements and compounds from approximate semi-analytical formulae M. -

Compton Sources of Electromagnetic Radiation∗
August 25, 2010 13:33 WSPC/INSTRUCTION FILE RAST˙Compton Reviews of Accelerator Science and Technology Vol. 1 (2008) 1–16 c World Scientific Publishing Company COMPTON SOURCES OF ELECTROMAGNETIC RADIATION∗ GEOFFREY KRAFFT Center for Advanced Studies of Accelerators, Jefferson Laboratory, 12050 Jefferson Ave. Newport News, Virginia 23606, United States of America kraff[email protected] GERD PRIEBE Division Leader High Field Laboratory, Max-Born-Institute, Max-Born-Straße 2 A Berlin, 12489, Germany [email protected] When a relativistic electron beam interacts with a high-field laser beam, the beam electrons can radiate intense and highly collimated electromagnetic radiation through Compton scattering. Through relativistic upshifting and the relativistic Doppler effect, highly energetic polarized photons are radiated along the electron beam motion when the electrons interact with the laser light. For example, X-ray radiation can be obtained when optical lasers are scattered from electrons of tens of MeV beam energy. Because of the desirable properties of the radiation produced, many groups around the world have been designing, building, and utilizing Compton sources for a wide variety of purposes. In this review paper, we discuss the generation and properties of the scattered radiation, the types of Compton source devices that have been constructed to present, and the future prospects of radiation sources of this general type. Due to the possibilities to produce hard electromagnetic radiation in a device small compared to the alternative storage ring sources, it is foreseen that large numbers of such sources may be constructed in the future. Keywords: Compton backscattering, Inverse Compton source, Thomson scattering, X-rays, Spectral brilliance 1. -
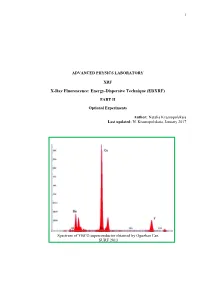
Advanced Physics Laboratory
1 ADVANCED PHYSICS LABORATORY XRF X-Ray Fluorescence: Energy-Dispersive Technique (EDXRF) PART II Optional Experiments Author: Natalia Krasnopolskaia Last updated: N. Krasnopolskaia, January 2017 Spectrum of YBCO superconductor obtained by Oguzhan Can. SURF 2013 2 Contents Introduction ………………………………………………………………………………………3 Experiment 1. Quantitative elemental analysis of coins ……………..………………………….12 Experiment 2. Study of Si PIN detector in the x-ray fluorescence spectrometer ……………….16 Experiment 3. Secondary enhancement of x-ray fluorescence and matrix effects in alloys.……24 Experiment 4. Verification of stoichiometry formula for superconducting materials..………….29 3 INTRODUCTION In Part I of the experiment you got familiar with fundamental ideas about the origin of x-ray photons as a result of decelerating of electrons in an x-ray tube and photoelectric effect in atoms of the x-ray tube and a target. For practical purposes, the processes in the x-ray tube and in the sample of interest must be explained in detail. An X-ray photon from the x-ray tube incident on a target/sample can be either absorbed by the atoms of the target or scattered. The corresponding processes are: (a) photoelectric effect (absorption) that gives rise to characteristic spectrum emitted isotropically; (b) Compton effect (inelastic scattering; energy shifts toward the lower value); and (c) Rayleigh scattering (elastic scattering; energy of the photon stays unchanged). Each event of interaction between the incident particle and an atom or the other particle is characterized by its probability, which in spectroscopy is expressed through the cross section σ of the process. Actually, σ has a dimension of [L2] and in atomic and nuclear physics has a unit of barn: 1 barn = 10-24cm2. -

Remote Sensing
remote sensing Article A Refined Four-Stream Radiative Transfer Model for Row-Planted Crops Xu Ma 1, Tiejun Wang 2 and Lei Lu 1,3,* 1 College of Earth and Environmental Sciences, Lanzhou University, Lanzhou 730000, China; [email protected] 2 Faculty of Geo-Information Science and Earth Observation (ITC), University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands; [email protected] 3 Key Laboratory of Western China’s Environmental Systems (Ministry of Education), Lanzhou University, Lanzhou 730000, China * Correspondence: [email protected]; Tel.: +86-151-1721-8663 Received: 17 March 2020; Accepted: 14 April 2020; Published: 18 April 2020 Abstract: In modeling the canopy reflectance of row-planted crops, neglecting horizontal radiative transfer may lead to an inaccurate representation of vegetation energy balance and further cause uncertainty in the simulation of canopy reflectance at larger viewing zenith angles. To reduce this systematic deviation, here we refined the four-stream radiative transfer equations by considering horizontal radiation through the lateral “walls”, considered the radiative transfer between rows, then proposed a modified four-stream (MFS) radiative transfer model using single and multiple scattering. We validated the MFS model using both computer simulations and in situ measurements, and found that the MFS model can be used to simulate crop canopy reflectance at different growth stages with an accuracy comparable to the computer simulations (RMSE < 0.002 in the red band, RMSE < 0.019 in NIR band). Moreover, the MFS model can be successfully used to simulate the reflectance of continuous (RMSE = 0.012) and row crop canopies (RMSE < 0.023), and therefore addressed the large viewing zenith angle problems in the previous row model based on four-stream radiative transfer equations. -

The Optical Effective Attenuation Coefficient As an Informative
hv photonics Article The Optical Effective Attenuation Coefficient as an Informative Measure of Brain Health in Aging Antonio M. Chiarelli 1,2,* , Kathy A. Low 1, Edward L. Maclin 1, Mark A. Fletcher 1, Tania S. Kong 1,3, Benjamin Zimmerman 1, Chin Hong Tan 1,4,5 , Bradley P. Sutton 1,6, Monica Fabiani 1,3,* and Gabriele Gratton 1,3,* 1 Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA 2 Department of Neuroscience, Imaging and Clinical Sciences, University G. D’Annunzio of Chieti-Pescara, 66100 Chieti, Italy 3 Psychology Department, University of Illinois at Urbana-Champaign, Champaign, IL 61820, USA 4 Division of Psychology, Nanyang Technological University, Singapore 639818, Singapore 5 Department of Pharmacology, National University of Singapore, Singapore 117600, Singapore 6 Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA * Correspondence: [email protected] (A.M.C.); [email protected] (M.F.); [email protected] (G.G.) Received: 24 May 2019; Accepted: 10 July 2019; Published: 12 July 2019 Abstract: Aging is accompanied by widespread changes in brain tissue. Here, we hypothesized that head tissue opacity to near-infrared light provides information about the health status of the brain’s cortical mantle. In diffusive media such as the head, opacity is quantified through the Effective Attenuation Coefficient (EAC), which is proportional to the geometric mean of the absorption and reduced scattering coefficients. EAC is estimated by the slope of the relationship between source–detector distance and the logarithm of the amount of light reaching the detector (optical density). -

3 Scattering Theory
3 Scattering theory In order to find the cross sections for reactions in terms of the interactions between the reacting nuclei, we have to solve the Schr¨odinger equation for the wave function of quantum mechanics. Scattering theory tells us how to find these wave functions for the positive (scattering) energies that are needed. We start with the simplest case of finite spherical real potentials between two interacting nuclei in section 3.1, and use a partial wave anal- ysis to derive expressions for the elastic scattering cross sections. We then progressively generalise the analysis to allow for long-ranged Coulomb po- tentials, and also complex-valued optical potentials. Section 3.2 presents the quantum mechanical methods to handle multiple kinds of reaction outcomes, each outcome being described by its own set of partial-wave channels, and section 3.3 then describes how multi-channel methods may be reformulated using integral expressions instead of sets of coupled differential equations. We end the chapter by showing in section 3.4 how the Pauli Principle re- quires us to describe sets identical particles, and by showing in section 3.5 how Maxwell’s equations for electromagnetic field may, in the one-photon approximation, be combined with the Schr¨odinger equation for the nucle- ons. Then we can describe photo-nuclear reactions such as photo-capture and disintegration in a uniform framework. 3.1 Elastic scattering from spherical potentials When the potential between two interacting nuclei does not depend on their relative orientiation, we say that this potential is spherical. In that case, the only reaction that can occur is elastic scattering, which we now proceed to calculate using the method of expansion in partial waves.