Bovine Trypanosomosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

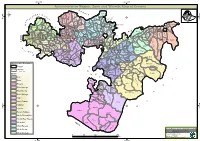
Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

Annual Report International Organization for Migration Special Liaison Office (IOM SLO) in Addis Ababa, Ethiopia
2015Annual Report International Organization for Migration Special Liaison Office (IOM SLO) in Addis Ababa, Ethiopia IOM OIM IOM PRESENCE In EthIOpIA IOM Presence in Ethiopia ETHIOPIA: Administrative Map (as of 14 January 2011) R ShireERITREA E Legend Tahtay Erob Laelay Adiyabo Mereb Ahferom Gulomekeda \\( Adiyabo Leke D National Capital Ganta Medebay Dalul North Adwa Afeshum Saesie Tahtay Zana Laelay Tsaedaemba Kafta Western Maychew PP Koraro Central Humera Asgede Tahtay Eastern Regional Capital Naeder Werei Hawzen Western Tsimbila Maychew Adet Leke Koneba Berahle Welkait Kelete Atsbi S Tigray Awelallo Wenberta International Boundary Tselemti Kola Degua Tsegede Mekele E Temben Temben P Addi Tselemt Tanqua Afdera Zone 2 Enderta Arekay Abergele Regional Boundary Tsegede Beyeda Ab Ala Mirab Saharti A Armacho Debark Samre Hintalo Erebti Abergele Wejirat Tach Megale Bidu Zonal Boundary Armacho Dabat Janamora Alaje Lay Sahla North Armacho Wegera Southern Ziquala Woreda Boundary Metema Gonder Sekota Endamehoni Raya Wag Azebo Chilga Yalo Amhara East Ofla Teru West Belesa Himra Kurri Gonder Dehana Belesa Lake Dembia Zuria Gaz Alamata Zone 4 Quara Gibla Semera Elidar Takusa Libo Ebenat Gulina Kemkem Bugna Lasta Kobo Awra Afar Gidan Lake Tana South (Ayna) 0 50 100 200 km Ewa Alfa Fogera Gonder North ¹ Lay Zone 1 Farta Meket Guba Lafto Dubti Gayint Asayta Semen Wollo P Jawi Achefer Tach Habru Chifra Bahr Dar East Wadla Delanta G U L F O F A D E N P Gayint Aysaita Creation date:14 Jan.2011 Dera Esite Bahirdar Ambasel Map Doc Name:21_ADM_000_ETH_011411_A0 -

FAO Quarterly Early Warning Bulletin for Food and Agriculture No
No. 17 October-December 2015 Quarterly Early Warning Bulletin for Food and Agriculture El Niño and Rainfall Source: International Research Institute for Climate and Society Alerts on threats to the food chain and food security in countries and regions FAO Quarterly Early Warning Bulletin for Food and Agriculture No. 17 October-December 2015 The Quarterly Early Warning Bulletin for Food and Agriculture integrates information on food security and threats to the food chain for the three months ahead. It is a product of collaboration between the Emergency Prevention System (EMPRES) for transboundary animal and plant pests and diseases and food safety threats, the Global Information and Early Warning System (GIEWS), and the Intelligence and Coordination Unit of the Food Chain Crisis Management Framework (FCC). Data is provided by GIEWS and EMPRES. Highlights Threats to the food chain1 and food security: Animal and aquatic diseases: o With the upcoming winter, the forecast for Avian Influenza is as follows: − In West Africa, the risk of further spread of Avian Influenza (H5N1 Highly Pathogenic Avian Influenza) to Burkina Faso, Côte d’Ivoire, Ghana, Niger and Nigeria in poultry is persisting. To date no reported cases of human samples tested. − In Southeast Asia and China, a high risk of occurrence of Avian Influenza (H5N1, H7N9, H5N2, H5N3, H5N6, and H5N8) outbreaks is expected with potential spread within endemic countries and to free areas. o The risk of re-occurrence and spread of a vector borne disease - Rift Valley fever (RVF) - is amplified in Eastern Africa with El Niño conditions. Strong persistent El Niño conditions are associated with periods of widespread and prolonged heavy rainfall creating ideal environmental and ecological conditions for the emergence of RVF vectors. -

Local History of Ethiopia : Daababali Kaaylu
Local History of Ethiopia Daababali Kaaylu - Dhera © Bernhard Lindahl (2008) Da.., see also De.. daaba (O) 1. honey comb; 2. stall in a market; 3. jamb, doorpost, support for wall; 4. ritual food etc. put aside for the gods; 5. poor, unfortunate JDG56 Daababali Kaaylu (D. Caailu) (area) 09/40 [+ WO] daad (Som) flood, floodwater; daadi (Som) to irrigate, to water, spill, scatter HDC83 Daadi 08/36 [WO] JCG97 Daalota, see Dalota HDM60 Daanci, see under Mendida 09/39 [WO] daar (Som) building of stone; daaro (Som) touch lightly, provoke HFE84 Daaro Tekle, see Dearo Tekle HBL91c Daas (small village with wells) 04/38 [20] -- Daasanach language (Daasanech), see Dasenech -- Daashi language, see Burji HDU17c Daawy, near Shewa Robit 10/39 [20] dab (Som) 1. fire; 2. trap, snare; (O) (Daab-) plant; (Dab-) hit a target; daba (O) 1. jamb, doorpost, support for wall; 2. ritual food etc put aside for the gods; 3. poor, unfortunate; daba (A) skin coloured yellowish, born by itinerant monks or by poor people; deba (däba) (A) intrigue Daba (Dabba), a male personal name HDK15 Daba (Dafu) (with church Medhane Alem) 09/37 [AA Gz] 0911'/3758' 2713 m, see under Ilfeta, cf Debe JDJ34 Daba 0923'/4158' 2098 m 09/41 [Gz] HDT29 Daba Ager, see Ahba H.... Daba Gabir (mountain in north-east Geralta) 13/39 [x] HET96 Daba Gebre Menfes K'idus (church) 1331'/3859', 13/38 [Gz] south of Abiy Adi HFE05 Daba Tadis 1349'/3859' 1569 m 13/38 [Gz] JCT21 Dabadur [=Daba Dur?] 0729'/4333' 922 m 07/43 [WO Gz] dabaka (O) tree, the bark of which gives red colour, Faurea rochetiana HCM91 Dabaka (Dabaca) 0712'/3926' 2209 m 07/39 [+ Gz] dabala (O) increase; daballe, dabballe (O) age grade 1-8 years in the Oromo gada system; dabaali (Som) cause to swim; debal (däbal) (A) 1. -
Urban Water Supply Universal Access Plan.Pdf
Federal Democratic Republic of Ethiopia Ministry of Water and Energy PART III Urban Water Supply Universal Access Plan (UWSPUAP) 2011-2015 December 2011 Addis Ababa 1 URBAN WATER SUPPLY UAP Table of Contents Executive Summary .............................................................................................................................................. 1 1. Introduction ................................................................................................................................................... 2 2. Background ................................................................................................................................................... 2 3. The Urban UAP model and Assumptions ..................................................................................................... 4 4. Sector policy and Strategy ............................................................................................................................. 7 5. Cross cutting Issues ..................................................................................................................................... 10 6. Physical Plan ............................................................................................................................................... 10 7. Financial Plan .............................................................................................................................................. 12 7.1. Financial plan for Project Implementation ......................................................................................... -

National Wash Program Selected Woredas and Small Towns
National WaSH Program Selected Woredas and Small Towns 1. Oromiya Region No Oromiya Woredas No Oromiya Woredas 41 Dugda 83 Ana Sora No Oromiya Woredas 42 Liban Chukala 84 Seba Boru 1 Digalu Tijo 43 Lume 85 Uraga 2 Gololcha 44 Bora 86 Odo Shakkiso 3 Jeju 45 Gudeya Bila 87 Liban 4 Limu Bilbilo 46 Guto Gida 88 Fentale 5 Tiyo 47 Sasiga 89 Babile 6 Zuway Dugda 48 Sibu Sire 90 Chinaksen 7 Adaba 49 Wayu Tuka 91 Qumbi 8 Gedeb Asasa 50 Seyo Nole 92 Guba Korcha 9 Agarfa 51 Nedjo 93 Chiro 10 Dawe Serer 52 Haru 94 Chewaka 11 Dinsho 53 Dale Sedi 95 Hurum 12 Gindhir 54 Dale Wabera 96 Darimu 13 Goro 55 Gawo Kebe 97 Dedo 14 Rayitu 56 Lalo Kile 98 Sokoru 15 Sinana 57 Seyo 99 Seka Chokorsa 16 Bedeno 58 Yubdo 100 Limu Seka 17 Gorogutu 59 Ayira 101 Ilu 18 Girawa 60 Guliso 102 Sodo Dachi 19 Kurfa Chale 61 Sude 103 Weliso 20 Kombolcha 62 Doddota 104 Ameya 21 Haromaya 63 Inkolo Wabe 105 Seden Soddo 22 Kersa 64 Robe 106 Nonno 23 Fedis 65 Lege Hidha 107 Dera 24 Boke 66 Sewena 108 Girar Jarso 25 Darulabu 67 G/Dhamole 109 Yaya Gulele 26 Doba 68 Siraro 110 Kuyu 27 Oda Bultum 69 Arsi Negele 111 Wuchale 28 Tulo 70 Kofele 112 Sululta 29 Hawi Gudina 71 Shashemene 113 Mulo 30 Burqa Dimtu 72 Kokosa 114 Sebeta Hawas 31 Becho 73 Akaki 115 Wolmera 32 Bedele 74 Teltale 116 Berek 33 Bilo Nopha 75 Dhas 117 Dendi 34 Didu 76 Yabello 118 Meta Robi 35 Metu 77 Arero 119 Abuna Ginde Beret 36 Yayu 78 Bule Hora 120 Ambo 37 Dorani 79 Gelana 121 Ilu Gelan 38 Adea 80 Moyale 122 Jibat 39 Adama 81 Hambela Wamana 123 Mida Kegn 40 AT/Jido Kombolcha 82 Kercha 124 Toke Kutaye National WaSH -

Prevalence of and Risk Factors For
Ophthalmic Epidemiology ISSN: 0928-6586 (Print) 1744-5086 (Online) Journal homepage: https://www.tandfonline.com/loi/iope20 Prevalence of and Risk Factors for Trachoma in Oromia Regional State of Ethiopia: Results of 79 Population-Based Prevalence Surveys Conducted with the Global Trachoma Mapping Project Berhanu Bero, Colin Macleod, Wondu Alemayehu, Solomon Gadisa, Ahmed Abajobir, Yilikal Adamu, Menbere Alemu, Liknaw Adamu, Michael Dejene, Addis Mekasha, Zelalem Habtamu Jemal, Damtew Yadeta, Oumer Shafi, Genet Kiflu, Rebecca Willis, Rebecca M. Flueckiger, Brian K. Chu, Alexandre L. Pavluck, Anthony W. Solomon & for the Global Trachoma Mapping Project To cite this article: Berhanu Bero, Colin Macleod, Wondu Alemayehu, Solomon Gadisa, Ahmed Abajobir, Yilikal Adamu, Menbere Alemu, Liknaw Adamu, Michael Dejene, Addis Mekasha, Zelalem Habtamu Jemal, Damtew Yadeta, Oumer Shafi, Genet Kiflu, Rebecca Willis, Rebecca M. Flueckiger, Brian K. Chu, Alexandre L. Pavluck, Anthony W. Solomon & for the Global Trachoma Mapping Project (2016) Prevalence of and Risk Factors for Trachoma in Oromia Regional State of Ethiopia: Results of 79 Population-Based Prevalence Surveys Conducted with the Global Trachoma Mapping Project, Ophthalmic Epidemiology, 23:6, 392-405, DOI: 10.1080/09286586.2016.1243717 To link to this article: https://doi.org/10.1080/09286586.2016.1243717 © 2016 The Authors. Published with license Published online: 07 Nov 2016. by Taylor & Francis Submit your article to this journal Article views: 2068 View Crossmark data Citing articles: 19 -

Summary and Statistical Report of the 2007 Population and Housing Census Results
Summary and Statistical Report of the 2007 Population and Housing Census Results Summary and Statistical Report of the 2007 Population and Housing Census Results 1 2 Summary and Statistical Report of the 2007 Population and Housing Census Results This document was printed by United Nations Population Fund(UNFPA) Summary and Statistical Report of the 2007 Population and Housing Census Results Summary and Statistical Report of the 2007 Population and Housing Census Results 3 Acknowledgements The third Population and Housing Census of Ethiopia was conducted in May and Novem- ber 2007. In accordance with the Census Commission Re-establishment Proclamation, the House of Peoples Representatives endorsed, on 4 December 2008, the census findings as organized by age, sex, religion, ethnicity, region and urban and rural residence. This sum- mary report contains an extract of the census data from this first release and the remaining census results will be disseminated subsequently. The Population and Housing Census which is a huge, complex and costly statistical opera- tion has been successfully completed through the concerted efforts of different government and non-government organizations, development partners, and individuals. The Office of the Census Commission and the Central Statistical Agency would, therefore, like to thank all who have contributed to the successful completion of the census. The Office of the Census Commission is very grateful to the Government of Ethiopia for its huge financial and administrative support. The Office is grateful to development partners particularly the United Nations Population Fund (UNFPA) and the Department for International Development (DfID) for their generous financial, logistics and technical support. -

Ethiopia: Oromia Region Administrative Map (As of 15 Aug 2017)
Oromia region administrative map (as of 15 Aug 2017) Ethiopia: ! ! ! ! ! ! ! ! ! ! ! ! ! ! Dera! ! BENISHANGUL GUMU ! ! ! ! ! Amuru ! ! ! ! AMHARA ! Ibantu Kiremu ! Goha Tsiyon ! Hinde ABORA ! ! ! ! Kiremu ! ! ! ! ! ! Wara Jarso Hidabu Abote ! ! ! ! Gida Ayana Jart!e Jardega Ejere ! AFAR ! Gelila ! Tulu Milki Mendi ! ! ! ! Haro Limu ! Ababo A!li Doro Deg!em ! ! ! Gida Ayana ! ! Gerar Jarso ! Mana Sibu Kiltu Kara Kuyu ! Limu Abay Chomen DEDU Wusha Gedel ! ! DIRE DAWA QILTU KARA Gori ! H!oro Abuna G/Beret ! ! ! Ginde Beret Fital Debre! Libanos ! ! ! ! ! ! Chinaksen Were Jiru Gutin Abe Dongoro Shambu Fincha !Kachise ! ! ! ! Abichuna Gne'a ! ! Nejo Yaya Gulele ! ! ! Jarso ! ! Wuchale ! Chinaksen Kokor ! KOMBOLCHA ! ! ! DEBEQA TOWN Nejo ! Muger ciminto Fabrika ! Chinaksen ! ! ! Kombolcha ! ! Bate Meta Robi ! !Kulu!bi ! !Melka Rafa ! Babo Guduru Adda Berga Jida Goro Gutu Chelenko !Adele ! Gudetu Kondole Jarso! Shuket Kembibit ! ! ! ! Begi Boji Dirmeji Jimma Genete ! ! ! ! Kersa Funyan Bira ! ! ! Bila Seyo ! ! ! ! ! ! Geba Dafeno Bila Guto Gida Hareto ! ! Boreda Meta Begi Chekorsa ! Sheno ! ! ! ! DederKobo ! Bila Jeldu ! ! Mulo ! Dawo Dengoro ! Biqiltu Qidame !Mulo ! ! ! Gursum ! ! Gojo Sululta ! ! Dob!a Boji Chekorsa ! Inchini Aleltu Mieso Deder Farda Babile Jere Jimma !Rare ! ! ! Hirna Doba ! Kurfa! Chele ! ! ! ! ! Sasi!ga Beke Sululta ! ! Mieso Hare WachaSoka HARERI ! Lalo A!sabi Goben ! ! Beke ! Tulo ! ! Gimbi ! Mida Kegn Ifata Sendafa! Debaso Girawa Boko Guliso Galo Gobu Seyo ! Asebot ! ! ! Inango ! ! Bedeno ! !Balemi Kora ! Gawo Kebe Ayira -

Ethiopia: Oromia Region Administrative Map (As of 05 Jan 2015)
Ethiopia: Oromia region administrative map (as of 05 Jan 2015) ! ! ! ! ! ! ! ! ! ! ! ! ! Gundo Meskel Town ! ! ! BENESHANGUL GUMU Dera ! ! ! ! ! Amuru ! ! ! ! AMHARA ! Ibantu Kiremu ! ABORA Goha Tsiyon Town ! ERITREA Hinde Town ! ! ! ! ! Kiremu Town ! ! ! ! ! Tulu Milki TownEjere Town ! Wara Jarso ! ! Alibo Town ! AFAR ! ! Gelila T!own ! Hidabu AboteDegem Mendi Town ! Jarte Jardega ! ! ! Haro Limu Gerbe Guracha TownHamibiso Town ! Ababo ! ! Fiche ! ! ! Mana Sibu Kiltu Kara Gida Ayana ! ! Abuna G/Beret Kuyu Ali Doro Town ! QILTU KARA TOWN Abay Chomen ! Limu DEDU TOWN North Shewa(R4) Wusha Gedel Town! Sekela ! Gori Town ! Horo Guduru ! Ginde Beret Debre Libanos ! ! ! Fital Town! ! ! Kachise! Town ! MENDIDA TOWN ! ! Jarso Chinaksen Nejo Gutin Town Horo Shambu Fincha ! ! DIRE DAWA Were Jiru Town ! ! ! Beke Qelate Town Muke Turi Town ! ! Chinaksen Yaya Gulele ! ! ! ! Nejo Town ! ! Chinaksen Kokor Town ! Abe Dongoro KOMBOLCHA TOWN ! Ijersa Goro ! ! DEBEQA TOWN ! Muger ciminto Fabrika Wuchale ! ! ! ! Boji Dirmeji MugerAdda Berga ! ! Bate Melka Rafa West Wellega Jimma Genete Guduru ! !Kulu!bi Adele ! ! ! Geba Dafeno Town Jida Goro Gutu ! ! ! Shuket TownMeta Robi Kembibit ! ! Kob!o ! Begi Town Babo Bila Town East Wellega Hareto Town ! ! ! ! KersaHaromaya Funyan Bira ! ! ! Bila Seyo! Sululta ! ! ! ! ! Jarso Chabi Town ! ! Boreda Begi Gudetu Kondole ! Sheno TownInchini ! ! ! ! Deder ! Bila TownBiqiltu QidameTown Jeldu ! ! Mulo Town ! WETER TOWNDawo Gursum Dengoro Town ! ! ! ! Doba Doba Town ! ! ! Guto Gida Gojo Town ! ! ! Sibu Sire Wayu Town ! Chancho