Enhanced Surveillance of Clostridium Difficile Infection: Ireland – Q4 2017 National Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DIRECTORY of HOSPITALS (And TREATMENT CENTRES)
COCT DIRECTORY OF HOSPITALS (and TREATMENT CENTRES) County Facility Name Facility Type Category Cover Type Additional information Cavan Cavan General Hospital, Cavan Public hospital Public 1 Clare Clare Mid Western Hospital, Ennis Public hospital Public 1 See notes (17) Clare Bushypark Treatment Centre, Ennis Private treatment centre Private 1 Covered for specified treatment programmes only. Cork Cork Bon Secours Hospital, Cork Private hospital Private 2 See notes (1)(8)(12)(13)(17)(26)(29)(33)(34)(35)(37)(38) Cork Cork University Hospital Public hospital Public 2 Cork Cork University Maternity Hospital Public hospital Public 2 Cork General Hospital, Bantry Public hospital Public 1 See notes (17) Cork General Hospital, Mallow Public hospital Public 1 See notes (17) Cork Mercy University Hospital Public hospital Public 2 See notes (17) Cork Mater Private Hospital, Cork Private hospital Private 2 See notes (5)(8)(10)(31) Cork South Infirmary/Victoria University Hospital Ltd. Public hospital Public 2 See notes (17) Cork Marymount Hospice Hospice Contact us for details Cork Tabor Lodge, Belgooly Private treatment centre Private 1 Covered for specified treatment programmes only. Donegal Donegal Letterkenny University Hospital Public hospital Public 1 Donegal White Oaks Rehabilitation Centre, Muff, Co. Donegal Private treatment centre Private 1 Covered for specified treatment programmes only. Dublin Dublin Beacon Hospital Private hospital Private 3 This hospital offers cardiac Level 2 (FPP) treatment. Dublin Beaumont Hospital (incorporating -

List of Approved Hospitals, Scan Centres & Treatment Centres
Cover For Me Cover For Us Cover For All Of Us Cover For Me Cover For Us Cover For All Of Us Hospital Maternity Out-Patient Activate Hospital & Core Plan Ranges Hospital Maternity Out-Patient Cover For Me Cover For Us Cover For All Of Us List Of Approved Hospitals, International Health & Travel Scan CentresSports Cover & Out-Patient Scan Treatment Centres September 2015 International Health & Travel Sports Cover Out-Patient Scan Hospital Maternity Out-Patient Women’s & Men's Health Complementary Therapy Dental & Optical Women’s & Men's Health Complementary Therapy Dental & Optical International Health & Travel Sports Cover Out-Patient Scan Women’s & Men's Health Complementary Therapy Dental & Optical 01 List Of Approved Hospitals Name of Hospital Type Cavan Cavan General Hospital Public Cover For Me Cover For Us Cover For All Of Us Clare Bushypark Treatment Centre, Ennis Addiction Centre Mid Western Regional Hospital, Ennis Public Cork Bantry General Hospital Public Bon Secours Hospital Private Cork University Hospital Public Hospital Maternity Out-Patient Cork University Maternity Hospital Public Cuan Mhuire, Farnanes Addiction Centre Mallow General Hospital Public Mater Private Cork Private Mercy University Hospital Public South Infirmary Victoria University Hospital Public St Mary’s Orthopaedic Hospital Public Tabor Lodge, Belgooly Addiction Centre International Health & Travel Sports Cover Out-Patient Scan Donegal Letterkenny General Hospital Public White Oaks Treatment Centre Addiction Centre Dublin Beacon Cancer Centre Private Beacon Hospital, Dublin 18 - Cardiac Procedures - All Plans High Tech - Private Beacon Hospital, Dublin 18 - All other procedures Private* Women’s & Men's Health Complementary Therapy Dental & Optical Beacon Hospital, Dublin 18 - Basic & Good Plans High Tech - Private * Beacon Hospital is classified as a private hospital (excluding cardiac procedures) for all plans in the Activate Hospital & Core plan ranges apart from Basic plan, Good plan & Activate Hospital plan. -

Intern Network Region Hospital Site
Intern Network Hospital Site Region Dublin Mid-Leinster Mater Misericordiae University Hospital, Eccles Street Dublin 7 Midland Regional Hospital, Arden Road,Tullamore, Co. Offaly Midland Regional Hospital, Ballyroan, Portlaoise, Co. Laois Midland Regional Hopsital, Mullingar, Co. Westmeath Cappagh Orthopaedic Hospital, Cappagh Rd, Northside, Dublin 11 Coombe Primary Care, 1 St. Catherine’s Lane West, Dublin 8 Beacon Hospital, Beacon Court, Bracken Road, Sandyford Industrial Estate, Dublin 18 St. Colmcilles' Hospital, Loughlinstown, Co. Dublin, D18 Mater Private Hospital, Eccles St, Northside, Dublin 7 St. Vincents University Hospital, 196 Merrion Road, Elm Park, Dublin St. Michaels' Hospital, George's Street Lower, Dún Laoghaire, Dublin Greystones Primary Care, Victoria Road, Greystones, Co. Wicklow Temple Street CHI, Temple St, Rotunda, Dublin 1 St. Vincents' Fairview, Convent Ave, Fairview, Drumcondra, Dublin 3 Dublin North East Connolly Hospital, Mill Rd, Abbotstown, Dublin, D15 University Hospital Waterford, Dunmore Road, Waterford, Our Lady of Lourdes Hospital, Drogheda Beaumont Hospital, Beaumont Rd, Beaumont, Dublin Temple Street, CHI, Temple St, Rotunda, Dublin 1 Cappagh Orthopaedic Hospital, Cappagh Rd, Northside, Dublin 11 Dublin South East Naas General Hospital, Naas, Co Kildare Linn Dara, Ballyfermot Rd, Cherry Orchard, Dublin St. James' Hospital, James's St, Ushers, Dublin 8 St. Lukes' Hospital, Kilkenny, Freshford Road, Friarsinch, Kilkenny Tallaght Hospital, Tallaght, Dublin 24 Wexford General Hospital, Newtown Rd, Carricklawn, -

Healthmail: Connected Agencies Here Is a List of Hospitals and Health Agencies Connected Securely to Healthmail
Healthmail: connected agencies Here is a list of hospitals and health agencies connected securely to Healthmail. Health Service Executive Healthmail users can communicate securely with all HSE Hospitals, Primary Care Teams or health care professionals who have an @hse.ie email address. Voluntary Hospitals Beaumont Hospital, Dublin - @beaumont.ie Cappagh National Orthopaedic Hospital - @cappagh.ie Children’s Health Ireland at Crumlin - @olchc.ie and @olhsc.ie Children’s Health Ireland at Temple St - @cuh.ie Children’s Health Ireland at Tallaght - @tuh.ie Children’s Health Ireland at Connolly - @nchg.ie Clontarf Hospital (Incorporated Orthopaedic Hospital of Ireland) - @ioh.ie Coombe Women & Infants University Hospital - @coombe.ie Dublin Dental University Hospital - @dental.tcd.ie Leopardstown Park Hospital - @lph.ie Mater Public, Dublin - @mater.ie Marymount University Hospital and Hospice, Cork - @marymount.ie Mercy Hospital, Cork - @muh.ie Milford Care Centre, Limerick - @milfordcarecentre.ie National Maternity Hospital, Holles Street - @nmh.ie National Rehabilitation Hospital - @nrh.ie Our Lady’s Hospice, Harold’s Cross, Dublin - @olh.ie Peamount Hospital - @peamount.ie Rotunda Maternity Hospital, Dublin - @rotunda.ie Royal Victoria Eye and Ear Hospital - @rveeh.ie South Infirmary Victoria University Hospital, Cork - @sivuh.ie St Francis Hospice, Dublin - @sfh.ie St James's Hospital, Dublin - @stjames.ie St John’s Hospital, Limerick - @stjohnshospital.ie St Luke’s Hospital, Rathgar, Dublin - @slh.ie St Vincent’s Hospitals Group - @st-vincents.ie, -

EQI-National-Data-Report-Final.Pdf
CONJOINT BOARD IN IRELAND of the Royal College of Physicians and Royal College of Surgeons 1 CUSTOM Authors Prof Steve Patchett (Chair) Consultant Gastroenterologist, Beaumont Hospital, Dublin Mr Fiachra Cooke Consultant Colorectal Surgeon, University Hospital Waterford Dr Glen Doherty Consultant Gastroenterologist, St. Vincent's University Hospital Ms Ann Hanly Consultant Surgeon, St. Vincent’s University Hospital Ms Sharon Hough Advanced Nurse Practitioner, St. James’s Hospital Dr Jan Leyden Consultant Gastroenterologist, Mater Misericordiae Ms Debbie McNamara Consultant Surgeon, Beaumont Hospital, Dublin, Irish Representative Association of Coloproctology of Great Britain and Ireland Dr Subhasish Sengupta Consultant Gastroenterologist, Lourdes Hospital, Drogheda Dr Maeve Skelly Consultant Gastroenterologist, University Hospital Limerick Ms Sarah Treleaven Programme Manager, RCPI Mr Philip Ryan Quality Analyst, RCPI Mr Conor Canavan Project Executive, RCPI CONJOINT BOARD IN IRELAND of the Royal College of Physicians and Royal College of Surgeons 2 Hospitals Contributing to 2016 Dataset Bantry General Hospital Beaumont Hospital, Dublin Blackrock Clinic Bon Secours Hospital Bon Secours Hospital Cavan General Dublin Galway Hospital Connolly Hospital Cork University Galway Clinic Blanchardstown Hospital Letterkenny University Mallow General Louth County Hospital Hospital Hospital Mater Misericordiae Mater Private Hospital, Mercy University University Hospital Dublin Hospital Midland Regional Mid-Western Regional Mid-Western Regional Hospital, -

Urgent and Emergency Care Provision in Ireland
Urgent and emergency care provision in Ireland What is urgent and emergency care? Urgent and emergency care consists of all the services which contribute to the management of people when immediate care is sought for a health condition along with the processes in place for referring patients between services. When patients need immediate care they can enter the health system through a range of services and will often use more than one. Ideally these services would be highly co-ordinated to ensure the time to definitive care is reduced and waste such as inappropriate use of emergency departments is avoided. Current provision in Ireland A wide range of services, public and private, provide emergency and urgent care in Ireland. The services within the emergency and urgent care system (EUCS) include General Practice (GP) (including out-of-hours GP co-operatives), emergency departments (EDs), urgent care centres, acute medical units (AMUs), minor injury units, ambulance services (provided by Dublin Fire Brigade and the National Ambulance Service), and pharmacies. When patients need immediate care, they can enter the health system through a range of services and will often use more than one in a single episode of illness. For example, they may phone an out-of-hours GP, be transferred by ambulance to an ED and be admitted to hospital. The combination of these services are defined as an EUCS. The provision of effective emergency and urgent care is critically dependent on all elements of the EUCS of a healthcare system (figure 1). A well-performing EUCS should improve the chances of survival in a patient with an emergency condition and an urgent condition should ideally be managed by a well- performing EUCS without admission to an inpatient bed. -

Where Are RCPI Webcast Centres Located?
Where are RCPI webcast centres located? Webcast Centres 2018-2019 Viewing Locations Altnagelvin Hospital Lecture Theatre 1, Centre for Medical and Dental Education and Training Bantry General Hospital Conference room or phone Bon Secours Tralee New Boardroom Cavan Monaghan Hospital RCSI Room Cork University Hospital Infusion Unit Meeting Room Craigavon Area Hospital Tutorial Room 1 MEC Curragh Military Hospital Lecture Hall, Military Medical School Ennis Hospital Library, Nurses Home Galway Clinic Boardroom First Floor Letterkenny University Hospital Large Conference Room Marymount University Hospital & Hospice Auditorium Mayo University Hospital NCHD Room Mercy University Hospital First Floor drawing room Midlands Regional Hospital, Portlaoise Conference Room, Library Midlands Regional Hospital, Tullamore Lecture theatre 2, Scott building Naas General Hospital Tutorial room/Education room Navan Hospital Conference centre Nenagh Hospital Conference Room Our Lady's Hospital, Navan Conference hall Portiuncula University Hospital Galway Room 6, centre of education Regional Hospital Mullingar Seminar Room Royal Victoria Hospital, Dublin Royal Victoria hospital Sligo University Hospital Lecture Theatre, Level 6 South Tipperary General Hospital, Clonmel Education room 1 at STGH - as usual South West Acute Hospital Room 1, Education Centre St. Luke’s Hospital, Kilkenny Tutorial room 2, Education building University Hospital Kerry Main Conference room University Hospital Limerick A tutorial room in the Clinical Education & Research Centre Wexford General Hospital Third floor conference room Who to Contact If you have any questions about our webcasts or how to become an RCPI Webcast Centre please contact our Postgraduate Medical Education Centre at [email protected] . -

Beaumont Hospital Annual Report 2008
Beau mont Hospi Beaumont Hospital Annual Report 2008 | Beaumont Hospital Annual Report 2008 ■ Contents Chairman’s Report 2 Chief Executive’s Review 6 Finance Report 16 Medical Administration Department 26 Director of Nursing Report 30 Hospital Divisions 44 Division of Surgery 44 Division of Medicine 48 Department of Anaesthesia and Intensive Care 62 Department of Radiology 66 Neuroscience Division 70 Division of Laboratory Medicine 74 Clinical Services Division 80 Non-Clinical Services Division 100 Functional/Support Departments 106 St. Joseph’s Hospital, Raheny 120 RCSI Education & Research Centre Annual Report 2008 124 Research and Publications/Presentations 132 Celebrating 21 Years 144 1 Chairman’s Report We are committed to maintaining and enhancing our quality performance and to play a leading role in patient care and in the transformation of our national health service. 2 Beaumont Hospital Annual Report 2008 | Chairman’sReport ■ Chairman’s Report Beaumont Hospital continues to commitment to carry out a leading role in the provision of provide to international standards colorectal, urological, head and neck, and upper GI surgery. a wide range of medical services Work has already begun on the preparation for the building in more than 50 specialties, to of our new Radiation Oncology centre, one of four such our local, regional and national units, which together will provide the national service. catchment populations, and to Other units will be located at St James’s Hospital, Dublin, engage in extensive research and and at the University Hospitals in Cork and Galway. The educational programmes. We do radiotherapy service which will be provided at Beaumont this in an environment of rapidly will enable us to provide an integrated comprehensive developing medical technology, Mr. -
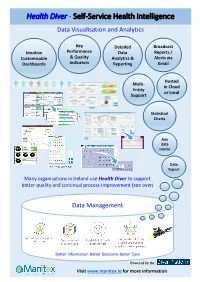
Health Diver - Self-Service Health Intelligence Data Visualisation and Analytics
Health Diver - Self-Service Health Intelligence Data Visualisation and Analytics Key Detailed Broadcast Intuitive Performance Data Reports / Customisable & Quality Analytics & Alerts via Dashboards Indicators Reporting Email Hosted Multi- in Cloud Entity or Local Support Statistical Charts Any data source Data Export Many organisations in Ireland use Health Diver to support better quality and continual process improvement (see over) Data Management Better Information Better Decisions Better Care Powered by the Visit www.manitex.ie for more information Self-Service Health Intelligence Experience CUSTOMERS EHR INFORMATION TOPIC Beaumont Hospital Patient Administration Blackrock Clinic - Patient Index Bon Secours Hospital, Cork - Inpatients & Daycase ADT Bon Secours Hospital, Dublin - Inpatients & Daycase Waiting List - Bed Management Bon Secours Hospital, Galway - Readmissions Bon Secours Hospital, Tralee - Delayed Discharges Bon Secours Hospital, Limerick - Outpatient Attendances Cavan General Hospital (HSE) - Outpatient Bookings Children's Health Ireland @ Temple St - Outpatient Waiting List Connolly Hospital, Blanchardstown (HSE) - HIPE Ely Hospital, Wexford (HSE) - Emergency Department Galway Clinic - Nursing Galway University Hospital (UHG & Merlin Park) (HSE) - Patient Workflow HSE Dublin North City & County (CHO9) Clinical Information & Departmental Systems HSE Dublin South, Kildare & W Wicklow (CHO7) - Order Communications Letterkenny General Hospital (HSE) - Radiology Louth County Hospital, Dundalk (HSE) - Laboratory Mater Misericordiae -

Difficile Infection: Ireland – Q3 2019 National Report
HSE-HPSC, Clostridioides difficile Enhanced Surveillance, Q3 2019 Report P a g e | 1 Enhanced Surveillance of Clostridioides (Clostridium) difficile Infection: Ireland – Q3 2019 National Report Executive Summary . During Q3 2019, a total of 582 cases of C. difficile infection (CDI) were reported to enhanced surveillance from 57 acute public and private hospitals across Ireland . The national overall rate of CDI in hospitalised patients was 4.5 cases per 10,000 bed days used (BDU) [465 cases], higher than that reported for Q3 2018 [403 cases; rate = 4.0] . There were 296 cases of CDI deemed to be hospital-acquired (HA-CDI), of which 268 were new, representing a national new HA-CDI rate of 2.6 [median rate = 1.6] . With regard to acquisition, while C. difficile was mostly associated with acute hospitals (296; 51%), there were many cases associated with the community (147; 25%) and long-term care facilities (LTCF) (46; 8%) . CDI symptom onset occurred in the community for 40% of all cases (232): o This emphasises the importance of considering CDI when evaluating any patient with potentially infectious diarrhoea in all healthcare settings, including hospitals, primary care and LTCF. Guidance on CDI for primary and long-term care settings is available at the following link: http://www.hpsc.ie/a- z/microbiologyantimicrobialresistance/clostridioidesdifficile/guidelines/File,14387,en.pdf o It also emphasises the importance for all microbiology laboratories in Ireland to implement the recommendations of the national C. difficile clinical guidelines to routinely include C. difficile testing for all faeces specimens that take the shape of the container submitted from patients aged ≥2 years, regardless of patient location or clinician request. -

The Net Range List of Approved Hospitals, Scan Centres & Treatment Centres April 2016
The Net Range List Of Approved Hospitals, Scan Centres & Treatment Centres April 2016 i 01 List Of Approved Hospitals Name of Hospital Type Cavan Cavan General Hospital Public ✓ ✓ ✓ Clare Bushypark Treatment Centre, Ennis Addiction Centre ✓ Mid Western Regional Hospital, Ennis Public ✓ ✓ ✓ Cork Bantry General Hospital Public ✓ ✓ ✓ Bon Secours Hospital, Cork Private ✓ ✓ Cork University Hospital Public ✓ ✓ ✓ Cork University Maternity Hospital Public ✓ ✓ ✓ Cuan Mhuire, Farnanes Addiction Centre ✓ Mallow General Hospital Public ✓ ✓ ✓ Mater Private Cork Private ✓ ✓ Mercy University Hospital Public ✓ ✓ ✓ South Infirmary Victoria University Hospital Public ✓ ✓ ✓ St Mary’s Orthopaedic Hospital Public Tabor Lodge, Belgooly Addiction Centre ✓ Donegal Letterkenny General Hospital Public ✓ ✓ ✓ White Oaks Treatment Centre Addiction Centre ✓ Dublin Beacon Hospital, Dublin 18 High Tech - Private See Table of Benefits Beacon Hospital Cancer Centre, Dublin 18 Private ✓ Beaumont Hospital, Dublin 9 Public ✓ ✓ ✓ Blackrock Clinic, Blackrock, Co Dublin High Tech - Private Blackrock Hospice (part cover only), Blackrock, Co Dublin Public ✓ ✓ ✓ 1 01 Hello And Welcome To GloHealth Name of Hospital Type Bon Secours Hospital, Glasnevin, Dublin 9 Private ✓ ✓ Cappagh National Orthopaedic Hospital, Dublin 11 Public Children's University Hospital, Temple St Public ✓ ✓ ✓ Connolly Hospital, Dublin 15 Public ✓ ✓ ✓ Coombe Women's and Infant's Hospital, Dublin 8 Public ✓ ✓ ✓ Hampstead Private Hospital, Dublin 9 Private ✓ Hermitage Medical Clinic, Dublin 20 Private ✓ ✓ Highfield Private Hospital, Dublin 9 Private ✓ Incorporated Orthopaedic Hospital of Ireland, Dublin 3 Public La Ginesa, St John of God, Stillorgan, Co. Dublin Private ✓ Mater Misericordiae University Hospital, Dublin 7 Public ✓ ✓ ✓ Mater Private Hospital, Dublin 7 High Tech - Private See Table of Benefits National Maternity Hospital, Dublin 2 Public ✓ ✓ ✓ Our Lady’s Hospice (part only), Harold’s Cross, Dublin 6W Public ✓ ✓ ✓ Our Lady's Hospital for Sick Children, Dublin 12 Public ✓ ✓ ✓ Peamount Hospital, Newcastle, Co. -

Directory of Hospitals (And Treatment Centres)
Directory of Hospitals (and Treatment Centres) How to use this directory: The directory below summarises the various approved hospitals and treatment centres we cover. These are organised by type of facility and category cover type. The categories are not a reflection of the level or quality of care provided. To determine the level of cover for a particular hospital and treatment centre, check the facility type, find the category cover type from the directory below and refer to your Table of Benefits. We have organised the directory by county to make it easier for you to find those near you. The directory changes from time to time. Please refer to www.vhi.ie/downloads for the most up-to-date list. FACILITY TYPE CATEGORY ADDITIONAL INFORMATION Hospitals (and Treatment Centres) COVER TYPE CAVAN HOSPITALS General Hospital, Cavan ■ Public Hospital Public 1 CLARE HOSPITALS Cahercalla Community Hospital Private hospital Private 1 Mid-Western Hospital, Ennis ■ Public hospital Public 1 OTHER MEDICAL FACILITIES Bushypark Treatment Centre, Ennis Private treatment centre Private 1 Covered for specified treatment programmes only CORK HOSPITALS Bon Secours Hospital, Cork ● Private hospital Private 2 Cork University Hospital Public hospital Public 2 Cork University Maternity Hospital Public hospital Public 2 General Hospital, Bantry ■ Public hospital Public 1 General Hospital, Mallow ■ Public hospital Public 1 Mercy University Hospital ■ Public hospital Public 2 Shanakiel Hospital ▲ Private hospital Private 2 South Infirmary/Victoria University Hospital Ltd. ■ Public hospital Public 2 St. Mary’s Orthopaedic Hospital ■ Public hospital Public 1 OTHER MEDICAL FACILITIES Marymount Hospice Hospice Contact us for details Tabor Lodge, Belgooly Private treatment centre Private 1 Covered for specified treatment programmes only DONEGAL HOSPITALS General Hospital, Letterkenny ■ Public hospital Public 1 OTHER MEDICAL FACILITIES White Oaks Rehabilitation Centre, Muff, Co.