Retention of Spliceosomal Components Along Ligated Exons Ensures Efficient Removal of Multiple Introns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differential Analysis of Gene Expression and Alternative Splicing
i bioRxiv preprint doi: https://doi.org/10.1101/2020.08.23.234237; this version posted August 24, 2020. The copyright holder for this preprint i (which was not certified by peer review)“main” is the author/funder, — 2020/8/23 who has — granted 9:38 — bioRxiv page a1 license — #1 to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. i i EmpiReS: Differential Analysis of Gene Expression and Alternative Splicing Gergely Csaba∗, Evi Berchtold*,y, Armin Hadziahmetovic, Markus Gruber, Constantin Ammar and Ralf Zimmer Department of Informatics, Ludwig-Maximilians-Universitat¨ Munchen,¨ 80333, Munich, Germany ABSTRACT to translation. Different transcripts of the same gene are often expressed at the same time, possibly at different abundance, While absolute quantification is challenging in high- yielding a defined mixture of isoforms. Changes to this throughput measurements, changes of features composition are further referred to as differential alternative between conditions can often be determined with splicing (DAS). Various diseases can be linked to DAS high precision. Therefore, analysis of fold changes events (3). Most hallmarks of cancer like tumor progression is the standard method, but often, a doubly and immune escape (4, 5) can be attributed to changes in differential analysis of changes of changes is the transcript composition (6). DAS plays a prominent role required. Differential alternative splicing is an in various types of muscular dystrophy (MD) (7), splicing example of a doubly differential analysis, i.e. fold intervention by targeted exon removal is a therapeutic option changes between conditions for different isoforms in Duchenne MD (8). -
Primepcr™Assay Validation Report
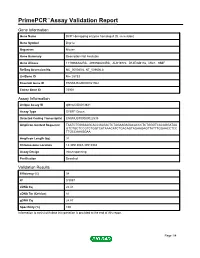
PrimePCR™Assay Validation Report Gene Information Gene Name DCP1 decapping enzyme homolog A (S. cerevisiae) Gene Symbol Dcp1a Organism Mouse Gene Summary Description Not Available Gene Aliases 1110066A22Rik, 4930568L04Rik, AU019772, D14Ertd817e, Mitc1, SMIF RefSeq Accession No. NC_000080.6, NT_039606.8 UniGene ID Mm.28733 Ensembl Gene ID ENSMUSG00000021962 Entrez Gene ID 75901 Assay Information Unique Assay ID qMmuCID0013841 Assay Type SYBR® Green Detected Coding Transcript(s) ENSMUST00000022535 Amplicon Context Sequence TAATCTGGGAAGCACCGAGACTCTAGAAGAGACACCCTCTGGGTCACAGGATAA GTCTGCTCCGTCTGGTCATAAACATCTGACAGTAGAAGAGTTATTTGGAACCTCC TTGCCAAAGGAA Amplicon Length (bp) 91 Chromosome Location 14:30513043-30518984 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) 98 R2 0.9997 cDNA Cq 22.41 cDNA Tm (Celsius) 81 gDNA Cq 24.87 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 1/4 PrimePCR™Assay Validation Report Dcp1a, Mouse Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 2/4 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Mouse Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. Detected Coding Transcript(s) This is a list of the Ensembl transcript ID(s) that this assay will detect. -

Exon Amplification: a Strategy to Isolate Mammalian Genes Based on RNA Splicing (Gene Cloning/Polymerase Chain Reaction) ALAN J
Proc. Natl. Acad. Sci. USA Vol. 88, pp. 4005-4009, May 1991 Genetics Exon amplification: A strategy to isolate mammalian genes based on RNA splicing (gene cloning/polymerase chain reaction) ALAN J. BUCKLER*, DAVID D. CHANG, SHARON L. GRAw, J. DAVID BROOK, DANIEL A. HABER, PHILLIP A. SHARP, AND DAVID E. HOUSMAN Center for Cancer Research, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139 Contributed by Phillip A. Sharp, January 25, 1991 ABSTRACT We have developed a method, exon amplifi- (7, 8). Thus, this method may be generally applicable for the cation, for fast and efficient isolation of coding sequences from selection of exon sequences from any gene. The method is complex mammalian genomic DNA. This method is based on also both rapid and easily adapted to large scale experiments. the selection of RNA sequences, exons, which are flanked by A series of cloned genomic DNA fragments can be screened functional 5' and 3' splice sites. Fragments of cloned genomic within 1-2 weeks. The sensitivity of this method is high. DNA are inserted into an intron, which is flanked by 5' and 3' Genomic DNA segments of 20 kilobases (kb) or more can be splice sites of the human immunodeficiency virus 1 tat gene successfully screened in a single transfection by using a set contained within the plasmid pSPL1. COS-7 cells are trans- of pooled subclones. This method thus allows the rapid fected with these constructs, and the resulting RNA transcripts identification of exons in mammalian genomic DNA and are processed in vivo. Splice sites of exons contained within the should facilitate the isolation of a wide spectrum of genes of inserted genomic fragment are paired with splice sites of the significance in physiology and development. -

Mrna Turnover Philip Mitchell* and David Tollervey†
320 mRNA turnover Philip Mitchell* and David Tollervey† Nuclear RNA-binding proteins can record pre-mRNA are cotransported to the cytoplasm with the mRNP. These processing events in the structure of messenger proteins may preserve a record of the nuclear history of the ribonucleoprotein particles (mRNPs). During initial rounds of pre-mRNA in the cytoplasmic mRNP structure. This infor- translation, the mature mRNP structure is established and is mation can strongly influence the cytoplasmic fate of the monitored by mRNA surveillance systems. Competition for the mRNA and is used by mRNA surveillance systems that act cap structure links translation and subsequent mRNA as a checkpoint of mRNP integrity, particularly in the identi- degradation, which may also involve multiple deadenylases. fication of premature translation termination codons (PTCs). Addresses Cotransport of nuclear mRNA-binding proteins with mRNA Wellcome Trust Centre for Cell Biology, ICMB, University of Edinburgh, from the nucleus to the cytoplasm (nucleocytoplasmic shut- Kings’ Buildings, Edinburgh EH9 3JR, UK tling) was first observed for the heterogeneous nuclear *e-mail: [email protected] ribonucleoprotein (hnRNP) proteins. Some hnRNP proteins †e-mail: [email protected] are stripped from the mRNA at export [1], but hnRNP A1, Current Opinion in Cell Biology 2001, 13:320–325 A2, E, I and K are all exported (see [2]). Although roles for 0955-0674/01/$ — see front matter these hnRNP proteins in transport and translation have been © 2001 Elsevier Science Ltd. All rights reserved. reported [3•,4•], their affects on mRNA stability have been little studied. More is known about hnRNP D/AUF1 and Abbreviations AREs AU-rich sequence elements another nuclear RNA-binding protein, HuR, which act CBC cap-binding complex antagonistically to modulate the stability of a range of DAN deadenylating nuclease mRNAs containing AU-rich sequence elements (AREs) DSEs downstream sequence elements (reviewed in [2]). -

RNA Components of the Spliceosome Regulate Tissue- and Cancer-Specific Alternative Splicing
Downloaded from genome.cshlp.org on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press Research RNA components of the spliceosome regulate tissue- and cancer-specific alternative splicing Heidi Dvinge,1,2,4 Jamie Guenthoer,3 Peggy L. Porter,3 and Robert K. Bradley1,2 1Computational Biology Program, Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington 98109, USA; 2Basic Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington 98109, USA; 3Human Biology Division, Fred Hutchinson Cancer Research Center, Seattle, Washington 98109, USA Alternative splicing of pre-mRNAs plays a pivotal role during the establishment and maintenance of human cell types. Characterizing the trans-acting regulatory proteins that control alternative splicing has therefore been the focus of much research. Recent work has established that even core protein components of the spliceosome, which are required for splicing to proceed, can nonetheless contribute to splicing regulation by modulating splice site choice. We here show that the RNA components of the spliceosome likewise influence alternative splicing decisions. Although these small nuclear RNAs (snRNAs), termed U1, U2, U4, U5, and U6 snRNA, are present in equal stoichiometry within the spliceosome, we found that their relative levels vary by an order of magnitude during development, across tissues, and across cancer samples. Physiologically relevant perturbation of individual snRNAs drove widespread gene-specific differences in alternative splic- ing but not transcriptome-wide splicing failure. Genes that were particularly sensitive to variations in snRNA abundance in a breast cancer cell line model were likewise preferentially misspliced within a clinically diverse cohort of invasive breast ductal carcinomas. -

Supplemental Information
Supplemental information Dissection of the genomic structure of the miR-183/96/182 gene. Previously, we showed that the miR-183/96/182 cluster is an intergenic miRNA cluster, located in a ~60-kb interval between the genes encoding nuclear respiratory factor-1 (Nrf1) and ubiquitin-conjugating enzyme E2H (Ube2h) on mouse chr6qA3.3 (1). To start to uncover the genomic structure of the miR- 183/96/182 gene, we first studied genomic features around miR-183/96/182 in the UCSC genome browser (http://genome.UCSC.edu/), and identified two CpG islands 3.4-6.5 kb 5’ of pre-miR-183, the most 5’ miRNA of the cluster (Fig. 1A; Fig. S1 and Seq. S1). A cDNA clone, AK044220, located at 3.2-4.6 kb 5’ to pre-miR-183, encompasses the second CpG island (Fig. 1A; Fig. S1). We hypothesized that this cDNA clone was derived from 5’ exon(s) of the primary transcript of the miR-183/96/182 gene, as CpG islands are often associated with promoters (2). Supporting this hypothesis, multiple expressed sequences detected by gene-trap clones, including clone D016D06 (3, 4), were co-localized with the cDNA clone AK044220 (Fig. 1A; Fig. S1). Clone D016D06, deposited by the German GeneTrap Consortium (GGTC) (http://tikus.gsf.de) (3, 4), was derived from insertion of a retroviral construct, rFlpROSAβgeo in 129S2 ES cells (Fig. 1A and C). The rFlpROSAβgeo construct carries a promoterless reporter gene, the β−geo cassette - an in-frame fusion of the β-galactosidase and neomycin resistance (Neor) gene (5), with a splicing acceptor (SA) immediately upstream, and a polyA signal downstream of the β−geo cassette (Fig. -

The Differential Interaction of Snrnps with Pre-Mrna Reveals Splicing Kinetics in Living Cells
Published October 4, 2010 This article has original data in the JCB Data Viewer JCB: Article http://jcb-dataviewer.rupress.org/jcb/browse/3011 The differential interaction of snRNPs with pre-mRNA reveals splicing kinetics in living cells Martina Huranová,1 Ivan Ivani,1 Aleš Benda,2 Ina Poser,3 Yehuda Brody,4,5 Martin Hof,2 Yaron Shav-Tal,4,5 Karla M. Neugebauer,3 and David StanČk1 1Institute of Molecular Genetics and 2J. Heyrovský Institute of Physical Chemistry, Academy of Sciences of the Czech Republic, 142 20 Prague, Czech Republic 3Max Planck Institute for Molecular Cell Biology and Genetics, 01307 Dresden, Germany 4The Mina and Everard Goodman Faculty of Life Sciences and 5Institute for Nanotechnology and Advanced Materials, Bar-Ilan University, Ramat Gan 52900, Israel recursor messenger RNA (pre-mRNA) splicing is Core components of the spliceosome, U2 and U5 snRNPs, catalyzed by the spliceosome, a large ribonucleo- associated with pre-mRNA for 15–30 s, indicating that protein (RNP) complex composed of five small nuclear splicing is accomplished within this time period. Additionally, P Downloaded from RNP particles (snRNPs) and additional proteins. Using live binding of U1 and U4/U6 snRNPs with pre-mRNA oc- cell imaging of GFP-tagged snRNP components expressed curred within seconds, indicating that the interaction of at endogenous levels, we examined how the spliceosome individual snRNPs with pre-mRNA is distinct. These results assembles in vivo. A comprehensive analysis of snRNP are consistent with the predictions of the step-wise model dynamics in the cell nucleus enabled us to determine of spliceosome assembly and provide an estimate on the snRNP diffusion throughout the nucleoplasm as well as rate of splicing in human cells. -

Sequences at the Exon-Intron Boundaries* (Split Gene/Mrna Splicing/Eukaryotic Gene Structure) R
Proc. Nati. Acad. Sci. USA Vol. 75, No. 10, pp. 4853-4857, October 1978 Biochemistry Ovalbumin gene: Evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries* (split gene/mRNA splicing/eukaryotic gene structure) R. BREATHNACH, C. BENOIST, K. O'HARE, F. GANNON, AND P. CHAMBON Laboratoire de Genetique Mol6culaire des Eucaryotes du Centre National de la Recherche Scientifique, Unite 44 de l'Institut National de la Sant6 et de la Recherche MWdicale, Institut de Chimie Biologique, Facult6 de Melecine, Strasbourg 67085, France Communicated by A. Frey-Wyssling, July 31, 1978 ABSTRACT Selected regions of cloned EcoRI fragments the 5' end of ov-mRNA and have revealed some interesting of the chicken ovalbumin gene have been sequenced. The po- features in the DNA sequences at exon-intron boundaries. sitions where the sequences coding for ovalbumin mRNA (ov- mRNA) are interrupted in the genome have been determined, and a previously unreported interruption in the DNA sequences MATERIALS AND METHODS coding for the 5' nontranslated region of the messenger has been discovered. Because directly repeated sequences are found at Plasmid pCR1 ov 2.1 containing the ov-ds-cDNA insert was exon-intron boundaries, the nucleotide sequence alone cannot prepared as described (9). EcoRI fragments "b," "c," and "d" define unique excision-ligation points for the processing of a previously cloned in X vectors (3) were transferred to the plas- possible ov-mRNA precursor. However, the sequences in these mid pBR 322. An EcoRI/HindIII of the EcoRI boundary regions share common features; this leads to the fragment proposal that there are, in fact, unique excision-ligation points fragment "a" containing the entirety of exon 7 (Fig. -

Splicing Graphs and RNA-Seq Data
Splicing graphs and RNA-seq data Herv´ePag`es Daniel Bindreither Marc Carlson Martin Morgan Last modified: January 2019; Compiled: May 19, 2021 Contents 1 Introduction 1 2 Splicing graphs 2 2.1 Definitions and example . .2 2.2 Details about nodes and edges . .2 2.3 Uninformative nodes . .5 3 Computing splicing graphs from annotations 6 3.1 Choosing and loading a gene model . .6 3.2 Generating a SplicingGraphs object . .6 3.3 Basic manipulation of a SplicingGraphs object . .8 3.4 Extracting and plotting graphs from a SplicingGraphs object . 12 4 Splicing graph bubbles 15 4.1 Some definitions . 15 4.2 Computing the bubbles of a splicing graph . 17 4.3 AScodes ............................................... 18 4.4 Tabulating all the AS codes for Human chromosome 14 . 19 5 Counting reads 20 5.1 Assigning reads to the edges of a SplicingGraphs object . 22 5.2 How does read assignment work? . 23 5.3 Counting and summarizing the assigned reads . 25 6 Session Information 26 1 Introduction The SplicingGraphs package allows the user to create and manipulate splicing graphs [1] based on annotations for a given organism. Annotations must describe a gene model, that is, they need to contain the following information: The exact exon/intron structure (i.e., genomic coordinates) for the known transcripts. The grouping of transcripts by gene. Annotations need to be provided as a TxDb or GRangesList object, which is how a gene model is typically represented in Bioconductor. 1 The SplicingGraphs package defines the SplicingGraphs container for storing the splicing graphs together with the gene model that they are based on. -

Nuclear PTEN Safeguards Pre-Mrna Splicing to Link Golgi Apparatus for Its Tumor Suppressive Role
ARTICLE DOI: 10.1038/s41467-018-04760-1 OPEN Nuclear PTEN safeguards pre-mRNA splicing to link Golgi apparatus for its tumor suppressive role Shao-Ming Shen1, Yan Ji2, Cheng Zhang1, Shuang-Shu Dong2, Shuo Yang1, Zhong Xiong1, Meng-Kai Ge1, Yun Yu1, Li Xia1, Meng Guo1, Jin-Ke Cheng3, Jun-Ling Liu1,3, Jian-Xiu Yu1,3 & Guo-Qiang Chen1 Dysregulation of pre-mRNA alternative splicing (AS) is closely associated with cancers. However, the relationships between the AS and classic oncogenes/tumor suppressors are 1234567890():,; largely unknown. Here we show that the deletion of tumor suppressor PTEN alters pre-mRNA splicing in a phosphatase-independent manner, and identify 262 PTEN-regulated AS events in 293T cells by RNA sequencing, which are associated with significant worse outcome of cancer patients. Based on these findings, we report that nuclear PTEN interacts with the splicing machinery, spliceosome, to regulate its assembly and pre-mRNA splicing. We also identify a new exon 2b in GOLGA2 transcript and the exon exclusion contributes to PTEN knockdown-induced tumorigenesis by promoting dramatic Golgi extension and secretion, and PTEN depletion significantly sensitizes cancer cells to secretion inhibitors brefeldin A and golgicide A. Our results suggest that Golgi secretion inhibitors alone or in combination with PI3K/Akt kinase inhibitors may be therapeutically useful for PTEN-deficient cancers. 1 Department of Pathophysiology, Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Shanghai Jiao Tong University School of Medicine (SJTU-SM), Shanghai 200025, China. 2 Institute of Health Sciences, Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences and SJTU-SM, Shanghai 200025, China. -

POLYADENYLATION REGULATION of U1A Mrna: CHARACTERIZING
STUDIES OF POLYADENYLATION REGULATION OF U1A mRNA BY AN RNP COMPLEX CONTAINING U1A AND U1 snRNP By ROSE MARIE CARATOZZOLO A dissertation submitted to the Graduate School – New Brunswick Rutgers, The State University of New Jersey and The Graduate School of Biomedical Sciences University of Medicine and Dentistry of New Jersey In partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Biochemistry Written under the direction of Samuel I. Gunderson, Ph.D., And approved by _____________________________ _____________________________ _____________________________ _____________________________ New Brunswick, New Jersey January, 2011 ABSTRACT OF THE DISSERTATION STUDIES OF POLYADENYLATION REGULATION OF U1A mRNA BY AN RNP COMPLEX CONTAINING U1A AND U1 snRNP By Rose Marie Caratozzolo Dissertation Director: Samuel I. Gunderson, Ph.D. The 3’-end processing of nearly all eukaryotic pre-mRNAs comprises multiple steps which culminate in the addition of a poly(A) tail, which is essential for mRNA stability, translation, and export. Consequently, polyadenylation regulation is an important component of gene expression. One way to regulate polyadenylation is to inhibit the activity of a single poly(A) site, as exemplified by the U1A protein that negatively autoregulates itself by binding to a Polyadenylation Inhibitory Element (PIE) site within the 3’ UTR of its own pre-mRNA. U1 snRNP, which is primarily involved in splice site recognition, inhibits poly(A) site activity in papillomaviruses by binding to 5’ splice site-like sequences, which have recently been named “U1-sites”. Here, a recently identified U1-site in the human U1A 3'UTR is examined and shown to synergize with the adjacent PIE site to inhibit polyadenylation. -

Allosteric Regulation of U1 Snrnp by Splicing Regulatory Proteins Controls
Downloaded from rnajournal.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Allosteric Regulation of U1 snRNP by Splicing Regulatory Proteins Controls Spliceosomal Assembly. Hossein Shenasa1, Maliheh Movassat1, Elmira Forouzmand1 and Klemens J. Hertel1 1. Department of Microbiology and Molecular Genetics, University of California Irvine, Irvine, California Keywords: Spliceosomal Assembly, U1 snRNP, Splice Site Selection, Splicing Regulatory Proteins, Allosteric Regulation 1 Downloaded from rnajournal.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Abstract: Alternative splicing is responsible for much of the transcriptomic and proteomic diversity observed in eukaryotes and involves combinatorial regulation by many cis-acting elements and trans-acting factors. SR and hnRNP splicing regulatory proteins often have opposing effects on splicing efficiency depending on where they bind the pre-mRNA relative to the splice site. Position-dependent splicing repression occurs at spliceosomal E-complex, suggesting that U1 snRNP binds but cannot facilitate higher order spliceosomal assembly. To test the hypothesis that the structure of U1 snRNA changes during activation or repression, we developed a method to structure-probe native U1 snRNP in enriched conformations that mimic activated or repressed spliceosomal E- complexes. While the core of U1 snRNA is highly structured, the 5' end of U1 snRNA shows different SHAPE reactivities and psoralen crosslinking efficiencies depending on where splicing regulatory elements are located relative to the 5' splice site. A motif within the 5' splice site binding region of U1 snRNA is more reactive towards SHAPE electrophiles when repressors are bound, suggesting U1 snRNA is bound, but less base paired.