Persistent Biotic Interactions of a Gondwanan Conifer from Cretaceous Patagonia to Modern Malesia ✉ Michael P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
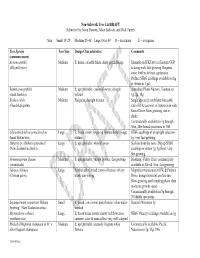
Recommended Non-Sidewalk Tree List DRAFT
Non-Sidewalk Tree List DRAFT Submitted by Jason Dewees, Mike Sullivan, and Dick Turner Size: Small 15-25’ Medium 25-40’ Large Over 40’ D = deciduous E = evergreen Tree Species Tree Size Design Characteristics Comments (common name) Acmena smithii Medium E; dense, colorful fruits; shiny green foliage Example on JFK Drive in Eastern GGP (lilly-pilly tree) is doing well; fast-growing. Requires some fertility for best appearance. Profuse SFBG seedlings available to dig or obtain in 1 gal. Banksia integrifolia Medium E; upright habit; creamy flowers; drought Australian Plants Nursery, Ventura, in (coast banksia) tolerant 1g, 5g, 15g Brahea edulis Medium Fan palm; drought tolerant Single specimen established on north (Guadalupe palm) side of JFK just west of intersection with Kezar Drive. Slow growing, sun or shade. Commercially available in 1g through 36in, 48in boxed specimens to 15ft Chiranthodendron pentadactylon Large E; broad crown; large red flowers, bold foliage SFBG seedlings of an upright selection: (hand flower tree) texture 1g; very fast-growing Hoheria sp. (Hoheria populnea? Large E; upright habit; white flowers Suckers from the roots. Dig up SFBG (New Zealand lacebark)) seedlings or obtain 1g, 5g there; very fast-growing Hymenosporum flavum Medium E; upright habit; yellow flowers; fast growing Boething, Valley Crest; commercially (sweetshade) available in 24in & 36in; fast-growing. Jubaea chilensis Large Feather palm; broad crown of leaves; silvery Magnificent specimen at JFK & Fuchsia (Chilean palm) trunk; sun-loving Drive; drought-tolerant, prefers sun. Slow-growing until trunking phase, then moderate growth speed. Commercially available in 5g through 20ft B&B specimens Leptospermum scoparium ‘Helene Small E; broad, low crown; pink flowers; often multi- Suncrest Nurseries 5g Strybing’ (New Zealand tea tree) trunked Metrosideros robusta Large E; broad dense crown; showy red flowers in SFBG Nursery seedlings available in 1g (northern rata) summer; slow & unusual but very well-adapted Michelia/Magnolia champaca or M. -

Report on Biodiversity and Tropical Forests in Indonesia
Report on Biodiversity and Tropical Forests in Indonesia Submitted in accordance with Foreign Assistance Act Sections 118/119 February 20, 2004 Prepared for USAID/Indonesia Jl. Medan Merdeka Selatan No. 3-5 Jakarta 10110 Indonesia Prepared by Steve Rhee, M.E.Sc. Darrell Kitchener, Ph.D. Tim Brown, Ph.D. Reed Merrill, M.Sc. Russ Dilts, Ph.D. Stacey Tighe, Ph.D. Table of Contents Table of Contents............................................................................................................................. i List of Tables .................................................................................................................................. v List of Figures............................................................................................................................... vii Acronyms....................................................................................................................................... ix Executive Summary.................................................................................................................... xvii 1. Introduction............................................................................................................................1- 1 2. Legislative and Institutional Structure Affecting Biological Resources...............................2 - 1 2.1 Government of Indonesia................................................................................................2 - 2 2.1.1 Legislative Basis for Protection and Management of Biodiversity and -

Fossil History of Curculionoidea (Coleoptera) from the Paleogene
geosciences Review Fossil History of Curculionoidea (Coleoptera) from the Paleogene Andrei A. Legalov 1,2 1 Institute of Systematics and Ecology of Animals, Siberian Branch, Russian Academy of Sciences, Ulitsa Frunze, 11, 630091 Novosibirsk, Novosibirsk Oblast, Russia; [email protected]; Tel.: +7-9139471413 2 Biological Institute, Tomsk State University, Lenin Ave, 36, 634050 Tomsk, Tomsk Oblast, Russia Received: 23 June 2020; Accepted: 4 September 2020; Published: 6 September 2020 Abstract: Currently, some 564 species of Curculionoidea from nine families (Nemonychidae—4, Anthribidae—33, Ithyceridae—3, Belidae—9, Rhynchitidae—41, Attelabidae—3, Brentidae—47, Curculionidae—384, Platypodidae—2, Scolytidae—37) are known from the Paleogene. Twenty-seven species are found in the Paleocene, 442 in the Eocene and 94 in the Oligocene. The greatest diversity of Curculionoidea is described from the Eocene of Europe and North America. The richest faunas are known from Eocene localities, Florissant (177 species), Baltic amber (124 species) and Green River formation (75 species). The family Curculionidae dominates in all Paleogene localities. Weevil species associated with herbaceous vegetation are present in most localities since the middle Paleocene. A list of Curculionoidea species and their distribution by location is presented. Keywords: Coleoptera; Curculionoidea; fossil weevil; faunal structure; Paleocene; Eocene; Oligocene 1. Introduction Research into the biodiversity of the past is very important for understanding the development of life on our planet. Insects are one of the Main components of both extinct and recent ecosystems. Coleoptera occupied a special place in the terrestrial animal biotas of the Mesozoic and Cenozoics, as they are characterized by not only great diversity but also by their ecological specialization. -

Agathis Macrophylla Araucariaceae (Lindley) Masters
Agathis macrophylla (Lindley) Masters Araucariaceae LOCAL NAMES English (pacific kauri); Fijian (da‘ua,dakua dina,makadri,makadre,takua makadre,dakua,dakua makadre) BOTANIC DESCRIPTION Agathis macrophylla is a tall tree typically to about 30–40 m tall, 3 m in bole diameter, with a broad canopy of up to 36 m diameter. Branches may be erect to horizontal and massive. Mature specimens have wide, spreading root systems whereas seedlings and young specimens have a vigorous taproot with one or more whorls of lateral roots. Leaves simple, entire, elliptic to lanceolate, leathery, and dark green, and shiny above and often glaucous below; about 7–15 cm long and 2–3.5 cm wide, with many close inconspicuous parallel veins. The leaves taper to a more or less pointed tip, rounded at the base, with the margins curved down at the edge. Petioles short, from almost sessile up to 5 mm long. Cones egg-shaped at the end of the first year, about 5 cm long, and 3 cm in diameter, more or less round at the end of the second year, 8–10 cm in diameter. Female cones much larger than males, globular, on thick woody stalks, green, slightly glaucous, turning brownish during ripening. Seeds brown, small, ovoid to globose, flattened, winged, and attached to a triangular cone scale about 2.5 cm across. BIOLOGY Pacific kauri is monoecious and produces cones instead of flowers. The first female cones begin to be produced at about 10 years old and take up to 2 years to mature (more often in 12-15 months). -

Baez NHM 1997.Pdf (3.622Mb)
HERP QL 668 B33 l':iif;,O0i.U'.Z06l("'^ ocxentxpc Papers Natural History Museum The University of Kansas 29 October 1997 Number 4:1^1 Redescription of the Paleogene Shelania pascuali from Patagonia and Its Bearing on the Relationships of Fossil and Recent Pipoid Frogs >. By o O Ana Maria BAez^ and Linda Trueb- o ^Departamento de Geologia, FacuUad de Ciencias Exactas, Universidad de 0) w >. > t- Buenos Aires, Pabellon II, Ciudad Universitaria, 1428 Buenos Aires, Argentina CO !-> >^ ^ £ -^ '^Division of Herpetology, Natural Histoiy Museum, and Department of P Kansas 66045-2454, J3 o, Systematics and Ecology, The University of Kansas, Lawrence, USA a o t3 o CO ^ t. CONTENTS ° CO I ABSTRACT 2 « RESUMEN 2 5S INTRODUCTION 2 Previous Paleontological Work 4 Acknowledgments 4 MATERIALS AND METHODS 5 General Methodology 5 Cladistic Methodology 5 Specimens Examined 6 STRATIGRAPHIC PROVENANCE AND AGE OF MATERIAL 6 REDESCRIPTION OF SHELANIA 8 ANALYSIS OF CHARACTERS 16 RESULTS 31 DISCUSSION 35 Taxonomic Considerations 35 Characters 36 LITERATURE CITED 37 APPENDIX 40 © Natural History Museum, The University of Kansas ISSN No. 1094-0782 — 2 Scientific Papers, Natural History Museum, The University of Kansas 1960, is redescribed on the basis of a series of 30 recently I ABSTIMCT Shdania pascuali Casamiquela, .'-\ ' . discovered specimens, which range in estimated snout-vent length from 30-100 mm, from the Paleo- y ' gene of Patagonia. This large pipoid anuran is distinguished by possessing a long, narrow braincase; an hourglass-shaped frontoparietal; a robust antorbital process on the edentate maxilla; long, straight {/) '/^ I ilia that describe a V-shape in dorsal profile; and a trunk that is long relative to the lengths of the head and limbs. -

Ecological Assessments in the B+WISER Sites
Ecological Assessments in the B+WISER Sites (Northern Sierra Madre Natural Park, Upper Marikina-Kaliwa Forest Reserve, Bago River Watershed and Forest Reserve, Naujan Lake National Park and Subwatersheds, Mt. Kitanglad Range Natural Park and Mt. Apo Natural Park) Philippines Biodiversity & Watersheds Improved for Stronger Economy & Ecosystem Resilience (B+WISER) 23 March 2015 This publication was produced for review by the United States Agency for International Development. It was prepared by Chemonics International Inc. The Biodiversity and Watersheds Improved for Stronger Economy and Ecosystem Resilience Program is funded by the USAID, Contract No. AID-492-C-13-00002 and implemented by Chemonics International in association with: Fauna and Flora International (FFI) Haribon Foundation World Agroforestry Center (ICRAF) The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. Ecological Assessments in the B+WISER Sites Philippines Biodiversity and Watersheds Improved for Stronger Economy and Ecosystem Resilience (B+WISER) Program Implemented with: Department of Environment and Natural Resources Other National Government Agencies Local Government Units and Agencies Supported by: United States Agency for International Development Contract No.: AID-492-C-13-00002 Managed by: Chemonics International Inc. in partnership with Fauna and Flora International (FFI) Haribon Foundation World Agroforestry Center (ICRAF) 23 March -

Using the Larval Parasitoid, Agathis Bishopi (Nixon) (Hymenoptera
Using the larval parasitoid, Agathis bishopi (Nixon) (Hymenoptera: Braconidae), for early detection of False Codling Moth, Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae) infested fruit Thesis submitted in fulfilment of the requirements for the degree of MASTER OF SCIENCE at RHODES UNIVERSITY by Kennedy Josaya Zimba December 2014 Abstract Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae) is one of the major citrus pests of economic importance for South Africa’s citrus industry. It is endemic to Africa, and therefore a phytosanitary pest with zero tolerance by most export markets. The cryptic nature of T. leucotreta makes visual inspection an inefficient method for detecting neonate larvae in fruit in the packhouse. Therefore, a more accurate method for sorting infested fruit at the packhouse, particularly for newly infested fruit could ensure market access. A recent study showed that fruit infested by T. leucotreta emit a chemical profile different from that of a healthy fruit. Several studies provide evidence that parasitoids locate their hosts feeding on fruit by exploiting the novel chemical profiles produced due to host herbivory. The aim of this study was to evaluate the potential of using the naturally occurring behaviour of a larval parasitoid Agathis bishopi (Nixon) (Hymenoptera: Braconidae) for detection of T. leucotreta infested fruit, by determining which compound in infested fruit is attractive to parasitoids. Y- tube olfactometer and flight-tunnel bioassays with healthy and T. leucotreta infested fruit showed a significantly stronger response of A. bishopi female parasitoids to infested fruit. Among the volatile compounds associated with T. leucotreta infested fruit, D-limonene elicited the strongest attraction to A. bishopi female parasitoids. -

Morphology and Anatomy of Pollen Cones and Pollen in Podocarpus Gnidioides Carrière (Podocarpaceae, Coniferales)
1 2 Bull. CCP 4 (1): 36-48 (6.2015) V.M. Dörken & H. Nimsch Morphology and anatomy of pollen cones and pollen in Podocarpus gnidioides Carrière (Podocarpaceae, Coniferales) Abstract Podocarpus gnidioides is one of the rarest Podocarpus species in the world, and can rarely be found in collections; fertile material especially is not readily available. Until now no studies about its reproductive structures do exist. By chance a 10-years-old individual cultivated as a potted plant in the living collection of the second author produced 2014 pollen cones for the first time. Pollen cones of Podocarpus gnidioides have been investigated with microtome technique and SEM. Despite the isolated systematic position of Podocarpus gnidioides among the other New Caledonian Podocarps, it shows no unique features in morphology and anatomy of its hyposporangiate pollen cones and pollen. Both the pollen cones and the pollen are quite small and belong to the smallest ones among recent Podocarpus-species. The majority of pollen cones are unbranched but also a few branched ones are found, with one or two lateral units each of them developed from different buds, so that the base of each lateral cone-axis is also surrounded by bud scales. This is a great difference to other coniferous taxa with branched pollen cones e.g. Cephalotaxus (Taxaceae), where the whole “inflorescence” is developed from a single bud. It could be shown, that the pollen presentation in the erect pollen cones of Podocarpus gnidioides is secondary. However, further investigations with more specimens collected in the wild will be necessary. Key words: Podocarpaceae, Podocarpus, morphology, pollen, cone 1 Introduction Podocarpus gnidioides is an evergreen New Caledonian shrub, reaching up to 2 m in height (DE LAUBENFELS 1972; FARJON 2010). -

Agathis Robusta and Agathis Australis Friends Friends
Plants in Focus, December 2016 Agathis robusta and Agathis australis Friends of GeelongBotanic Left: The Qld Kauri Agathis robusta, planted in the Albury BG in 1910, is the largest recorded in the Big Tree Register. Note gardener. [1] Right: The NZ Kauri Agathis australis, named Tane Mahuta (Lord of the Forest), in the Waipoua Forest is the largest known in NZ. Photo: Prof. Chen Hualin, CC BY-SA 4.0, zh.wikipedia.org Kauris (Agathis sp.) are conifers Conifers, along with the other Gymnosperms (Cycads and Ginkgoes), first appeared about 300 Ma (Million years ago) at the end of the Carboniferous when the world’s coal deposits were being laid down with the remains of the spore-producing trees of that period. The early conifers looked like modern Araucaria. These trees spread throughout the world and displaced their predecessors. The age of the seed plants had arrived. The conifers are a hardy lot. They survived the largest mass extinction the earth has known, 252 Ma, at the end of the Permian Period. But more challenges lay ahead. Sometime in the next 50 Myr (Million years) one of Gymnosperms gave rise to the flowering plants, the Angiosperms. By 100 Ma, in the Cretaceous period, Angiosperms were widespread. And so the battle began - and still continues to this day. The flowering plants have many features that make them more successful in many environments, so their take-over of many habitats was complete by about 65 Ma at the end of the age of the dinosaurs. But in the world’s harsh environments the conifers continue to not just survive, but flourish. -

Fossil Pollen Records Indicate That Patagonian Desertification Was Not Solely a Consequence of Andean Uplift
ARTICLE Received 25 Oct 2013 | Accepted 4 Mar 2014 | Published 28 Mar 2014 DOI: 10.1038/ncomms4558 Fossil pollen records indicate that Patagonian desertification was not solely a consequence of Andean uplift L. Palazzesi1,2, V.D. Barreda1, J.I. Cuitin˜o3, M.V. Guler4, M.C. Tellerı´a5 & R. Ventura Santos6 The Patagonian steppe—a massive rain-shadow on the lee side of the southern Andes—is assumed to have evolved B15–12 Myr as a consequence of the southern Andean uplift. However, fossil evidence supporting this assumption is limited. Here we quantitatively estimate climatic conditions and plant richness for the interval B10–6 Myr based on the study and bioclimatic analysis of terrestrially derived spore–pollen assemblages preserved in well-constrained Patagonian marine deposits. Our analyses indicate a mesothermal climate, with mean temperatures of the coldest quarter between 11.4 °C and 16.9 °C (presently B3.5 °C) and annual precipitation rarely below 661 mm (presently B200 mm). Rarefied richness reveals a significantly more diverse flora during the late Miocene than today at the same latitude but comparable with that approximately 2,000 km further northeast at mid-latitudes on the Brazilian coast. We infer that the Patagonian desertification was not solely a consequence of the Andean uplift as previously insinuated. 1 Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Angel Gallardo 470 (C1405DJR), Buenos Aires, Argentina. 2 Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, UK. 3 Universidad de Buenos Aires, Departamento de Ciencias Geolo´gicas, Facultad de Ciencias Exactas y Naturales. Intendente Gu¨iraldes 2160 (C1428EHA), Buenos Aires, Argentina. -

Forestry Department Food and Agriculture Organization of the United Nations
Forestry Department Food and Agriculture Organization of the United Nations Forest Health & Biosecurity Working Papers OVERVIEW OF FOREST PESTS BRAZIL January 2007 Forest Resources Development Service Working Paper FBS/11E Forest Management Division FAO, Rome, Italy Forestry Department Overview of forest pests - Brazil DISCLAIMER The aim of this document is to give an overview of the forest pest1 situation in Brazil. It is not intended to be a comprehensive review. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. © FAO 2007 1 Pest: Any species, strain or biotype of plant, animal or pathogenic agent injurious to plants or plant products (FAO, 2004). ii Overview of forest pests - Brazil TABLE OF CONTENTS Introduction..................................................................................................................... 1 Forest pests...................................................................................................................... 1 Naturally regenerating forests..................................................................................... 1 Insects ..................................................................................................................... 1 Diseases.................................................................................................................. -

Insects and Related Arthropods Associated with of Agriculture
USDA United States Department Insects and Related Arthropods Associated with of Agriculture Forest Service Greenleaf Manzanita in Montane Chaparral Pacific Southwest Communities of Northeastern California Research Station General Technical Report Michael A. Valenti George T. Ferrell Alan A. Berryman PSW-GTR- 167 Publisher: Pacific Southwest Research Station Albany, California Forest Service Mailing address: U.S. Department of Agriculture PO Box 245, Berkeley CA 9470 1 -0245 Abstract Valenti, Michael A.; Ferrell, George T.; Berryman, Alan A. 1997. Insects and related arthropods associated with greenleaf manzanita in montane chaparral communities of northeastern California. Gen. Tech. Rep. PSW-GTR-167. Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Dept. Agriculture; 26 p. September 1997 Specimens representing 19 orders and 169 arthropod families (mostly insects) were collected from greenleaf manzanita brushfields in northeastern California and identified to species whenever possible. More than500 taxa below the family level wereinventoried, and each listing includes relative frequency of encounter, life stages collected, and dominant role in the greenleaf manzanita community. Specific host relationships are included for some predators and parasitoids. Herbivores, predators, and parasitoids comprised the majority (80 percent) of identified insects and related taxa. Retrieval Terms: Arctostaphylos patula, arthropods, California, insects, manzanita The Authors Michael A. Valenti is Forest Health Specialist, Delaware Department of Agriculture, 2320 S. DuPont Hwy, Dover, DE 19901-5515. George T. Ferrell is a retired Research Entomologist, Pacific Southwest Research Station, 2400 Washington Ave., Redding, CA 96001. Alan A. Berryman is Professor of Entomology, Washington State University, Pullman, WA 99164-6382. All photographs were taken by Michael A. Valenti, except for Figure 2, which was taken by Amy H.