DIGESTIVE SYSTEM -2 Emma Jakoi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coffee and Its Effect on Digestion
Expert report Coffee and its effect on digestion By Dr. Carlo La Vecchia, Professor of Medical Statistics and Epidemiology, Dept. of Clinical Sciences and Community Health, Università degli Studi di Milano, Italy. Contents 1 Overview 2 2 Coffee, a diet staple for millions 3 3 What effect can coffee have on the stomach? 4 4 Can coffee trigger heartburn or GORD? 5 5 Is coffee associated with the development of gastric or duodenal ulcers? 6 6 Can coffee help gallbladder or pancreatic function? 7 7 Does coffee consumption have an impact on the lower digestive tract? 8 8 Coffee and gut microbiota — an emerging area of research 9 9 About ISIC 10 10 References 11 www.coffeeandhealth.org May 2020 1 Expert report Coffee and its effect on digestion Overview There have been a number of studies published on coffee and its effect on different areas of digestion; some reporting favourable effects, while other studies report fewer positive effects. This report provides an overview of this body of research, highlighting a number of interesting findings that have emerged to date. Digestion is the breakdown of food and drink, which occurs through the synchronised function of several organs. It is coordinated by the nervous system and a number of different hormones, and can be impacted by a number of external factors. Coffee has been suggested as a trigger for some common digestive complaints from stomach ache and heartburn, through to bowel problems. Research suggests that coffee consumption can stimulate gastric, bile and pancreatic secretions, all of which play important roles in the overall process of digestion1–6. -

Calcium-Sensing Receptor Is a Physiologic Multimodal Chemosensor Regulating Gastric G-Cell Growth and Gastrin Secretion
Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion Jianying Fenga, Clark D. Petersena, David H. Coyb, Jian-Kang Jiangc, Craig J. Thomasc, Martin R. Pollakd, and Stephen A. Wanka,1 aDigestive Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892-1804; bPeptide Research Laboratories, Department of Medicine, Tulane Health Sciences Center, New Orleans, LA 70112-2699; cNational Institutes of Health Chemical Genomics Center, National Human Genome Research Institute, National Institutes of Health, Bethesda, MD 20892; and dDivision of Nephrology, Beth Israel Deaconess Medical Center, Boston, MA 02215 Edited* by Martha Vaughan, National Heart, Lung, and Blood Institute, Bethesda, MD, and approved September 1, 2010 (received for review June 25, 2010) The calcium-sensing receptor (CaR) is the major sensor and The sensing of extracellular Ca2+ and the regulation of Ca2+ regulator of extracellular Ca2+, whose activity is allosterically reg- homestasis has been largely attributed to the calcium-sensing ulated by amino acids and pH. Recently, CaR has been identified in receptor (CaR), a member of the C family of G protein-coupled the stomach and intestinal tract, where it has been proposed to receptors (GPCR). Consistent with this function, CaR is ex- 2+ function in a non-Ca2+ homeostatic capacity. Luminal nutrients, pressed on extracellular Ca -regulating cells such as para- such as Ca2+ and amino acids, have been recognized for decades thyroid, thyroid parafollicular, renal tubular, and bone cells (6). as potent stimulants for gastrin and acid secretion, although the The CaR has a large NH2 terminal extracellular domain molecular basis for their recognition remains unknown. -

Parietal Cell Hyperplasia Induced by Long-Term Administration of Antacids to Rats
Gut: first published as 10.1136/gut.19.9.798 on 1 September 1978. Downloaded from Gut, 1978, 19, 798-801 Parietal cell hyperplasia induced by long-term administration of antacids to rats G. MAZZACCA1, F. CASCIONE, G. BUDILLON, L. D'AGOSTINO, L. CIMINO, AND C. FEMIANO From the Division of Gastroenterology, 2nd School ofMedicine, University ofNaples, and the Department ofPathology, Institute for Tumoral Diseases, Naples, Italv SUMMARY Suspension of magnesium and aluminum hydroxide (30-60 mEq/24 h) or a comparable volume of water was orally administered by gastric intubation to two groups of 20 male wistar rats each over 60 days. The antacid treatment led to a significant increase in the height (0-464 + 0-02 mm v. 0-318 ± 0 06) and in the volume (472 ± 32 mm3 v. 328 ± 45) of the fundic mucosa ofthe stomach, in the average count of parietal cells per unit area of the mucosa (32-37 ± 1 8 v. 22-3 + 1 6), and in the total parietal cell population of the stomach (53-6 + 3.5 x 106 v. 43-2 + 3.7 x 106). Furthermore fasting serum gastrin concentration was significantly higher in the antacid treated rats (81.2 + 7.4 pg/ml) than in control animals (56-9 ± 6-9 pg/ml). It is known that a feedback mechanism governs the The animals of group B received each time and by relationship between antral gastrin release and the same procedure a comparable volume of water. intraluminal gastric pH (Walsh et al., 1975). During the night each animal was housed in a Furthermore, gastrin exerts a trophic influence on separate cage and the antacid suspension was the the gastric mucosa with increase in the parietal cell only drinking fluid available to the rats of group A. -

Overview of Gastrointestinal Function
Overview of Gastrointestinal Function George N. DeMartino, Ph.D. Department of Physiology University of Texas Southwestern Medical Center Dallas, TX 75390 The gastrointestinal system Functions of the gastrointestinal system • Digestion • Absorption • Secretion • Motility • Immune surveillance and tolerance GI-OP-13 Histology of the GI tract Blood or Lumenal Serosal Side or Mucosal Side Structure of a villus Villus Lamina propria Movement of substances across the epithelial layer Tight junctions X Lumen Blood Apical membrane Basolateral membrane X X transcellular X X paracellular GI-OP-19 Histology of the GI tract Blood or Lumenal Serosal Side or Mucosal Side Motility in the gastrointestinal system Propulsion net movement by peristalsis Mixing for digestion and absorption Separation sphincters Storage decreased pressure GI-OP-42 Intercellular signaling in the gastrointestinal system • Neural • Hormonal • Paracrine GI-OP-10 Neural control of the GI system • Extrinsic nervous system autonomic central nervous system • Intrinsic (enteric) nervous system entirely with the GI system GI-OP-14 The extrinsic nervous system The intrinsic nervous system forms complete functional circuits Sensory neurons Interneurons Motor neurons (excitatory and inhibitory) Parasympathetic nerves regulate functions of the intrinsic nervous system Y Reflex control of gastrointestinal functions Vago-vagal Afferent reflex Salivary Glands Composition of Saliva O Proteins α−amylase lactoferrin lipase RNase lysozyme et al mucus O Electrolyte solution water Na+ , K + - HCO3 -

Cellular Localization of Gastric Inhibitory Polypeptide in the Duodenum and Jejunum
Gut: first published as 10.1136/gut.14.4.284 on 1 April 1973. Downloaded from Gut, 1973, 14, 284-288 Cellular localization of gastric inhibitory polypeptide in the duodenum and jejunum JULIA M. POLAK, S. R. BLOOM', MARION KUZIO, J. C. BROWN, AND A. G. E. PEARSE From the Department ofHistochemistry, Royal Postgraduate Medical School, Hammersmith Hospital, London, and the Department ofPhysiology, University ofBritish Columbia, Vancouver, Canada SUMMARY Indirect immunofluorescence studies using an antiserum to purified porcine gastric inhibitory polypeptide indicate, in the gastrointestinal tract of dog and man, that this polypeptide is present in cells situated predominantly in the mid-zone of the glands in the duodenum and, to a lesser extent, in the jejunum. Absolute correlation of the gastric inhibitory polypeptide cell with one or other of the known endocrine-like cells identified by electron microscopy awaits confirmation by electron immunocytochemistry. It is here identified as an endocrine polypeptide cell of the APUD series and, provisionally, as the D, cell. While the hormonal status of a given polypeptide depends ultimately on physiological experiments the present results strengthen the view that gastric inhibitory polypeptide is indeed a hormone. In 1969 Brown, Pederson, Jorpes, and Mutt described We report here the results of immunofluorescence an enterogastrone, extractable from porcine intestine, studies on the localization of GIP in human and http://gut.bmj.com/ which strongly inhibited gastric acid secretion. The canine intestine. purification of an apparently similar enterogastrone was reported in the same year by Lucien, Itoh, Sun, Material and Methods Meyer, Carlton, and Schally. The first of these poly- peptides, named gastric inhibitory polypeptide Operative samples of duodenal and jejunal mucosa (GIP) by Brown, Mutt, and Pederson (1970), was from seven human subjects were studied. -

Expression of Cell Membrane Complement Regulatory Glycoproteins Along the Normal and Diseased Human Gastrointestinal Tract
522 Gut 1998;42:522–529 Expression of cell membrane complement regulatory glycoproteins along the normal and diseased human gastrointestinal tract Gut: first published as 10.1136/gut.42.4.522 on 1 April 1998. Downloaded from A E Berstad, P Brandtzaeg Abstract tract. Indeed, activated complement has been Background/Aims—Uncontrolled comple- detected in lesions of inflammatory bowel ment activation may be of immunopatho- disease,2–4 coeliac disease,5 and recently also in logical importance in inflammatory Helicobacter pylori gastritis.6 diseases of the gastrointestinal tract. Ex- Certain complement activation products, pression of membrane bound factors that notably the C3b fragment and the C5b-7 com- regulate complement activation was there- plex, can on their own—that is, without associ- fore studied in situ. ated antibody—bind to any nearby cell Methods—Frozen tissue specimens were membrane.7 Therefore it is crucial that com- obtained from patients with Helicobacter plement activity is tightly regulated. Mam- pylori gastritis, coeliac disease, Crohn’s malian cells are protected from complement disease, or ulcerative colitis, and from induced damage by a family of cell membrane histologically normal controls. Sections complement regulatory glycoproteins that were examined by immunofluorescence downregulate activation of homologous com- with monoclonal antibodies to protectin plement on their cell surface.8 Protectin (CD59), decay accelerating factor (DAF), (CD59) is broadly distributed on cells of and membrane cofactor protein (MCP). haemopoietic and non-haemopoietic origin79; Results—Protectin and MCP were widely it inhibits the formation of terminal comple- expressed in normal and diseased mu- ment complex by preventing the binding of C9 cosae. -

Somatostatin Inhibits Gastric Acid Secretion After Gastric Mucosal Prostaglandin Synthesis Inhibition by Indomethacin in Man
Gut: first published as 10.1136/gut.26.11.1189 on 1 November 1985. Downloaded from Gut, 1985, 26, 1189-1191 Somatostatin inhibits gastric acid secretion after gastric mucosal prostaglandin synthesis inhibition by indomethacin in man M H MOGARD, V MAXWELL, T KOVACS, G VAN DEVENTER, J D ELASHOFF, T YAMADA, G L KAUFFMAN JR, AND J H WALSH From the Centerfor Ulcer Research and Education, VA Wadsworth MedicallSurgical Services and UCLA, LosAngeles, California, USA. SUMMARY The inhibitory effect of indomethacin, 200+200 mg administered per os over 24 hours, on the prostaglandin E2 generative capacity of gastric mucosal tissue was determined in healthy male volunteers. The effect of prostaglandin synthesis inhibition on somatostatin induced suppression of food-stimulated acid secretion was tested. Peptone meal stimulated acid secretion was quantified in five healthy volunteers by intragastric titration with and without indomethacin pretreatment. Somatostatin doses of 200, 400, and 800 pmol/kg/h each significantly inhibited the peptone stimulated acid output. Indomethacin treatment, resulting in 90% inhibition of prostaglandin E2 synthesis, did not affect glucose- or peptone-stimulated acid output or modify the inhibitory action of somatostatin. Clinically, acid inhibition by somatostatin has been used to treat bleeding peptic ulcers. Ulcer haemorrhage may be preceded by an excessive use of drugs that inhibit prostaglandin synthesis such as aspirin or other non-steroidal anti-inflammatory agents. Recent observations in the rat indicate that prostaglandins mediate the inhibitory action of somatostatin on gastric acid secretion. The present results suggest that prostaglandins are not http://gut.bmj.com/ required for inhibition of gastric acid secretion by somatostatin in man. -
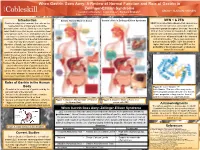
A Review of Normal Function and Role of Gastrin in Zollinger-Ellison
When Gastrin Goes Awry: A Review of Normal Function and Role of Gastrin in Zollinger-Ellison Syndrome Leigha LaTourette1, Janel Cross2, Barbara Brabetz2 Department of Plant and Animal Science1, Department of Natural Sciences and Mathematics2 Introduction Gastrin: Normal Mode of Action Gastrin’s Role in Zollinger-Ellison Syndrome MEN 1 & ZES Gastrin is a digestive hormone that acts on the MEN1 is an inheritable disease that causes over neuroendocrine and parietal cells of the secretion of hormones via tumors on the gastrointestinal tract to ultimately secrete gastric endocrine glands throughout the body.4 Around a acid. Gastrin secretion begins even before food 30% of these tumors are found to be malignant. is consumed, as the mere anticipation of a meal ZES is commonly associated with the MEN1 due can lead to a series of events ending in the to the presence of tumors found in the stomach creation of the gastric acid needed to breakdown and small intestine.4 These gastrointestinal foods. Not only does Gastrin support the tumors caused by MEN 1 give the person a digestion of foods, but it also stimulates growth, higher likelihood of contracting ZES, as the secretion, blood flow, and acts as a defense probability of having pancreatic or duodenal mechanism against bacteria in the tumors is increased.4 gastrointestinal system. When the production of Gastrin becomes overt, major consequences like that of Zollinger Ellison Syndrome (ZES). ZES is an extremely rare disease occurring in people between the ages of 30-60.4 ZES is related to the uncontrolled secretion of gastrin due to the presence of certain pancreatic or duodenal tumors. -

Reciprocal Regulation of Antral Gastrin and Somatostatin Gene Expression by Omeprazole-Induced Achlorhydria
Reciprocal regulation of antral gastrin and somatostatin gene expression by omeprazole-induced achlorhydria. S J Brand, D Stone J Clin Invest. 1988;82(3):1059-1066. https://doi.org/10.1172/JCI113662. Research Article Gastric acid exerts a feedback inhibition on the secretion of gastrin from antral G cells. This study examines whether gastrin gene expression is also regulated by changes in gastric pH. Achlorhydria was induced in rats by the gastric H+/K+ ATPase inhibitor, omeprazole (100 mumol/kg). This resulted in fourfold increases in both serum gastrin (within 2 h) and gastrin mRNA levels (after 24 h). Antral somatostatin D cells probably act as chemoreceptors for gastric acid to mediate a paracrine inhibition on gastrin secretion from adjacent G cells. Omeprazole-induced achlorhydria reduced D-cell activity as shown by a threefold decrease in antral somatostatin mRNA levels that began after 24 h. Exogenous administration of the somatostatin analogue SMS 201-995 (10 micrograms/kg) prevented both the hypergastrinemia and the increase in gastrin mRNA levels caused by omeprazole-induced achlorhydria. Exogenous somatostatin, however, did not influence the decrease in antral somatostatin mRNA levels seen with achlorhydria. These data, therefore, support the hypothesis that antral D cells act as chemoreceptors for changes in gastric pH, and modulates somatostatin secretion and synthesis to mediate a paracrine inhibition on gastrin gene expression in adjacent G cells. Find the latest version: https://jci.me/113662/pdf Reciprocal Regulation of Antral Gastrin and Somatostatin Gene Expression by Omeprazole-induced Achlorhydria Stephen J. Brand and Deborah Stone Departments ofMedicine, Harvard Medical School and Massachusetts General Hospital, Gastrointestinal Unit, Boston, Massachusetts Abstract Substantial evidence supports the hypothesis that gastric acid inhibits gastrin secretion through somatostatin released Gastric acid exerts a feedback inhibition on the secretion of from antral D cells (9, 10). -

Lecture Series Gastrointestinal Tract
Lecture series Gastrointestinal tract Professor Shraddha Singh, Department of Physiology, KGMU, Lucknow INNERVATION OF GIT • 1.Intrinsic innervation-1.Myenteric/Auerbach or plexus Local 2.Submucosal/Meissners plexus 2.Extrinsic innervation-1.Parasympathetic or -2.Sympathetic Higher centre Enteric Nervous System - Lies in the wall of the gut, beginning in the esophagus and - extending all the way to the anus - controlling gastrointestinal movements and secretion. - (1) an outer plexus lying between the longitudinal and circular muscle layers, called the myenteric plexus or Auerbach’s plexus, - controls mainly the gastrointestinal movements - (2) an inner plexus, called the submucosal plexus or Meissner’s plexus, that lies in the submucosa. - controls mainly gastrointestinal secretion and local blood flow Enteric Nervous System - The myenteric plexus consists mostly of a linear chain of many interconnecting neurons that extends the entire length of the GIT - When this plexus is stimulated, its principal effects are - (1) increased tonic contraction, or “tone,” of the gut wall, - (2) increased intensity of the rhythmical contractions, - (3) slightly increased rate of the rhythmical contraction, - (4) increased velocity of conduction of excitatory waves along the gut wall, causing more rapid movement of the gut peristaltic waves. - Inhibitory transmitter - vasoactive intestinal polypeptide (VIP) - pyloric sphincter, sphincter of the ileocecal valve Enteric Nervous System - The submucosal plexus is mainly concerned with controlling function within the inner wall - local intestinal secretion, local absorption, and local contraction of the submucosal muscle - Neurotransmitters: - (1) Ach (7) substance P - (2) NE (8) VIP - (3)ATP (9) somatostatin - (4) 5 – HT (10) bombesin - (5) dopamine (11) metenkephalin - (6) cholecystokinin (12) leuenkephalin Higher centre innervation - the extrinsic sympathetic and parasympathetic fibers that connect to both the myenteric and submucosal plexuses. -

A History of Gastric Secretion and Digestion a History of Gastric Secretion and Digestion Experimental Studies to 1975
A History of Gastric Secretion and Digestion A History of Gastric Secretion and Digestion Experimental Studies to 1975 HORACE W. DAVENPORT William Beaumont Professor of Physiology Emeritus The University of Michigan Springer New Y ork 1992 Copyright © 1992 by the American Physiological Society Originally published by American Physiological Society in 1992 Softcoverreprint of the bardeover 1st edition 1992 All rights reserved. No partoftbis publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission ofOxford University Press. Library ofCongress Cataloging-in-Publication Data Davenport, Horace Willard, 1912- A history of gastric secretion and digestion : experimental studiesto 1975 I Horace W. Davenport. p. cm. lncludes bibliographical references and index. ISBN 978-1-4614-7602-3 (eBook) DOI 10.1007/978-1-4614-7602-3 I. Gastroenterology-History. 2. Gastric-Secretion-Research-History. 3. Digestion-Research-History. I. Title. [DNLM: I. Digestion. 2. Gastric Acid-secretion. 3. Gastroenterology-history. 4. Research-history. 5. Stomach-chemistry. 6. Stomach-physiology. Wlll.l D247h] QP145.D325 1992 612.3'2'072-dc20 DNLM/DLC for Library ofCongress 91-31832 987654321 For Charles F. Code, known to every gastroenterologist as "Charlie Code" and as their preeminent physiologist for the last fifty years Preface For centuries men speculated about the process of gastric digestion, but Iate in the eighteenth and early in the nineteenth centuries physiologists, both physicians and laymen, began to accumulate experimental evidence about its nature. At the same time, others discovered that the stomach is capable of secreting a strong mineral acid, and the questions of how that secretion is produced and how it is controlled became enduring problems. -

Human Newborn Hypergastrinemia: an Investigation of Prenatal and Perinatal Factors and Their Effects on Gastrin
Pediat. Res. 12: 652-654 (1978) Acid secretion hypergastrinemia gastrin newborn Human Newborn Hypergastrinemia: An Investigation of Prenatal and Perinatal Factors and Their Effects on Gastrin ARTHUR R. EULER(13', MARVIN E. AMENT. AND JOHN H. WALSH Division of Gastroenterology, Departments of Pediatrics and Medicine, University of California, School of Medicine, Los Angeles, California, USA Summary stimulus for hydrochloric acid production by the parietal cells of the fundic mucosa (3). The release of gastrin from antral and Because gastrin is a potent gastric acid stimulus and gastric duodenal mucosa is augmented by multiple stimuli, including acid secretion begins soon after birth, we measured umbilical antral distension, vagal stimulation, catecholamines, polypep- cord serum gastrins. We also examined multiple factors present tides, and amino acids (11). We, therefore, determined gastrin during the gestation, labor, delivery, and immediate postpartum levels in the cord blood and concomitantly examined multiple period to see what effect, if any, these might have on the serum prenatal and perinatal factors to find what effect, if any, they gastrins. might have on the gastrin levels we found. Serum gastrin was Two groups were studied: 217 newborn infants and 802 adults also determined during the first hours of life while gastric acid without Zollinger-Ellison syndrome. The newborns' median secretion was monitored in the infants. serum gastrin was 100 pg/ml compared to the adult median of 39 pg/ml. The newborn mean was 135 pg/ml and the corre- sponding adult value was 40 pg/ml (P < 0.001). Twenty-nine MATERIALS AND METHODS newborns had gastrin determinations greater than 200 pg/ml; Two hundred seventeen infants had mixed venous and arterial five were greater than 500 pg/ml.