A Contextual Approach to the Study of Faunal Assemblages from Lower and Middle Palaeolithic Sites in the UK
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NM News Aug 15
THE NEEDHAM MARKET NEWSLETTER PUBLISHED BY NEEDHAM MARKET TOWN COUNCIL August 2015 - No 470 and Distributed Throughout Needham Market Free of Charge Needham Market Phoenix Youth Football Club 40th Year Celebrations 1977 See details on page 22 2014 www.needhammarkettc.co.uk TOWN COUNCIL Needham Market Town Council OFFICE TOWN CLERK: ASSISTANT TOWN CLERK: Town Council Office, Kevin Hunter Kelaine Spurdens Community Centre, School Street, LIST OF TOWN COUNCILLORS: TELEPHONE: Needham Market IP6 8BB BE ANNIS OBE Grinstead House, Grinstead Hill, NM IP6 8EY 01449 720531 (07927 007895) Telephone: 01449 722246. D CAMPBELL ‘Chain House’ 1 High Street, NM, IP6 8AL 01449 720952 An answerphone is in R CAMPBELL The Acorns, Hill House Lane, NM, IP6 8EA 01449 720729 operation when the office TS CARTER Danescroft, Ipswich Road, NM, IP6 8EG 01449 401325 is unmanned. RP DARNELL 27 Pinecroft Way, NM, IP6 8HB 07990 583162 The office is open to the JE LEA MA Town Mayor/Chair of Council public Mondays and 109 Jubilee Crescent, NM IP6 8AT 01449 721544 Thursdays 10am to 12noon. I MASON 114 Quinton Road, NM IP6 8TH 01449 721162 MG NORRIS 20 Stowmarket Road, NM, IP6 8DS 01449 720871 E.mail: KMN OAKES, 89 Stowmarket Road, NM, IP6 8ED 07702 339971 clerk@needhammarkettc. f9.co.uk S PHILLIPS 46 Crowley Road IP6 8BJ 01449 721710 S ROWLAND 9 Orchid Way, NM, IP6 8JQ 01449 721507 Web: D SPURLING 36 Drift Court, NM, IP6 8SZ 01449 723208 www.needhammarkettc. co.uk M SPURLING 36 Drift Court, NM, IP6 8SZ 01449 723208 X STANSFIELD Deputy Town Mayor/Deputy Chair of Council Town Council meetings Hope Cottage, 7 Stowmarket Road IP6 8DR 07538 058304 are held on the first and third Wednesdays of each AL WARD MBE 22 Chalkeith Road, NM, IP6 8HA 01449 720422 month at 7:25 p.m. -

Childcare Sufficiency Assessment (CSA) December 2019 – December 2020
Childcare Sufficiency Assessment (CSA) December 2019 – December 2020 Suffolk County Council Early Years and Childcare Service December 2019 Page 2 of 89 CONTENTS Table of Contents COVID – 19 5 1. Overall assessment and summary 5 England picture compared to Suffolk 6 Suffolk contextual information 6 Overall sufficiency in Suffolk 7 Deprivation 7 How Suffolk ranks across the different deprivation indices 8 2. Demand for childcare 14 Population of early years children 14 Population of school age children 14 3. Parent and carer consultation on childcare 15 4. Provision for children with special educational needs and disabilities 18 Number of children with special educational needs and disabilities (SEND) 18 5. Supply of childcare, Suffolk picture 20 Number of Early Years Providers 20 All Providers in Suffolk - LOP and Non LOP 20 Number of school age providers and places 21 6. Funded early education 22 Introduction to funded early education 22 Proportion of two year old children entitled to funded early education 22 Take up of funded early education 22 Comparison of take up of funded early education 2016 -2019 23 7. Three and four-year-old funded entitlement – 30hrs 24 30 hr codes used in Suffolk 25 Table 8 25 8. Providers offering funded early education places and places available. 26 Funded early education places available 26 Early education places at cluster level 28 9. Hourly rates 31 Hourly rate paid by Suffolk County Council 31 Hourly rate charged by providers 31 Mean hourly fee band for Suffolk 31 December 2019 Page 3 of 89 10. Quality of childcare 32 Ofsted inspection grades 32 11. -

Babergh District Council Work Completed Since April
WORK COMPLETED SINCE APRIL 2015 BABERGH DISTRICT COUNCIL Exchange Area Locality Served Total Postcodes Fibre Origin Suffolk Electoral SCC Councillor MP Premises Served Division Bildeston Chelsworth Rd Area, Bildeston 336 IP7 7 Ipswich Cosford Jenny Antill James Cartlidge Boxford Serving "Exchange Only Lines" 185 CO10 5 Sudbury Stour Valley James Finch James Cartlidge Bures Church Area, Bures 349 CO8 5 Sudbury Stour Valley James Finch James Cartlidge Clare Stoke Road Area 202 CO10 8 Haverhill Clare Mary Evans James Cartlidge Glemsford Cavendish 300 CO10 8 Sudbury Clare Mary Evans James Cartlidge Hadleigh Serving "Exchange Only Lines" 255 IP7 5 Ipswich Hadleigh Brian Riley James Cartlidge Hadleigh Brett Mill Area, Hadleigh 195 IP7 5 Ipswich Samford Gordon Jones James Cartlidge Hartest Lawshall 291 IP29 4 Bury St Edmunds Melford Richard Kemp James Cartlidge Hartest Hartest 148 IP29 4 Bury St Edmunds Melford Richard Kemp James Cartlidge Hintlesham Serving "Exchange Only Lines" 136 IP8 3 Ipswich Belstead Brook David Busby James Cartlidge Nayland High Road Area, Nayland 228 CO6 4 Colchester Stour Valley James Finch James Cartlidge Nayland Maple Way Area, Nayland 151 CO6 4 Colchester Stour Valley James Finch James Cartlidge Nayland Church St Area, Nayland Road 408 CO6 4 Colchester Stour Valley James Finch James Cartlidge Nayland Bear St Area, Nayland 201 CO6 4 Colchester Stour Valley James Finch James Cartlidge Nayland Serving "Exchange Only Lines" 271 CO6 4 Colchester Stour Valley James Finch James Cartlidge Shotley Shotley Gate 201 IP9 1 Ipswich -

MID SUFFOLK DISTRICT COUNCIL PARISH COUNCIL ELECTION Date : 3Rd May 2007
MID SUFFOLK DISTRICT COUNCIL PARISH COUNCIL ELECTION Date : 3rd May 2007 Parish Candidates Description Votes Cast Ashbocking Andrew Michael Gaught Farmer Elected Uncontested Tony Richard Gilbert Elected Robert Leggett Elected John Gordon Sinclair Pollard Engineer Elected Brian Colin Poole Elected Elizabeth Mary Stegman Elected Grahame Retired Lecturer Elected Tanner Ashfield Cum Thorpe Simon Geoffrey Edward Elected Garrett Uncontested Robert William Grimsey Elected Myles Gordon Elected Hansen Geoffrey Alan Hazlewood Elected Brian William Lennon Elected Bacton Robert James Black Elected Uncontested Bernard Gant Elected John Creasy Gooderham Elected Mary Esther Hawkins Elected Paul Dean Howlett Elected Roderick Paul Elected Wickenden Paul Elected Wigglesworth Badwell Ash Clive Frederick Bassett Elected Uncontested Angela Mary Brooks Elected Arthur George Diaper Elected Penny Frances Kirkby Elected Richard Pratt Elected David Smith Builder Elected Page 1 of 28 MID SUFFOLK DISTRICT COUNCIL PARISH COUNCIL ELECTION Date : 3rd May 2007 Parish Candidates Description Votes Cast Barham Neil Rayner Frederick Elected Cooper Uncontested Trevor David Girling Elected Jeremy Lea Elected Dorothy Lillian Blanche Mayhew Elected Gordon John Musson Elected Jan Elected Risebrow Helen Elizabeth Elected Whitefield Barking Steven Mark Independent Elected Austin Uncontested Michael Bailey Retired Local Government Officer Elected John Russell Tennant Berry Elected Alison Jane Emsden Elected Alan Kevin Jones Independent Elected Susan Margaret Elected Marsh Mike -

ELECTORAL DIVISION PROFILE 2017 This Division Comprises Eye, Fressingfield, Hoxne, Stradbroke and Laxfield Wards
HOXNE & EYE ELECTORAL DIVISION PROFILE 2017 This Division comprises Eye, Fressingfield, Hoxne, Stradbroke and Laxfield wards www.suffolkobservatory.info © Crown copyright and database rights 2017 Ordnance Survey 100023395 2 CONTENTS . Demographic Profile: Age & Ethnicity . Economy and Labour Market . Schools & NEET . Index of Multiple Deprivation . Health . Crime & Community Safety . Additional Information . Data Sources 3 ELECTORAL DIVISION PROFILES: AN INTRODUCTION These profiles have been produced to support elected members, constituents and other interested parties in understanding the demographic, economic, social and educational profile of their neighbourhoods. We have used the latest data available at the time of publication. Much more data is available from national and local sources than is captured here, but it is hoped that the profile will be a useful starting point for discussion, where local knowledge and experience can be used to flesh out and illuminate the information presented here. The profile can be used to help look at some fundamental questions e.g. Does the age profile of the population match or differ from the national profile? . Is there evidence of the ageing profile of the county in all the wards in the Division or just some? . How diverse is the community in terms of ethnicity? . What is the impact of deprivation on families and residents? . Does there seem to be a link between deprivation and school performance? . What is the breakdown of employment sectors in the area? . Is it a relatively healthy area compared to the rest of the district or county? . What sort of crime is prevalent in the community? A vast amount of additional data is available on the Suffolk Observatory www.suffolkobservatory.info The Suffolk Observatory is a free online resource that contains all Suffolk’s vital statistics; it is the one‐stop‐shop for information and intelligence about Suffolk. -

New Evidence for Complex Climate Change in MIS 11 from Hoxne, Suffolk, UK
ARTICLE IN PRESS Quaternary Science Reviews 27 (2008) 652–668 New evidence for complex climate change in MIS 11 from Hoxne, Suffolk, UK Nick Ashtona,Ã, Simon G. Lewisb, Simon A. Parfittc,d, Kirsty E.H. Penkmane, G. Russell Coopef aDepartment of Prehistory and Europe, British Museum, Franks House, 56 Orsman Road, London N1 5QJ, UK bDepartment of Geography, Queen Mary, University of London, Mile End Road, London E1 4NS, UK cDepartment of Palaeontology, Natural History Museum, Cromwell Road, London SW7 5BD, UK dInstitute of Archaeology, University College London, 31-34 Gordon Square, London WC1H 0PY, UK eBioArCh, Departments of Biology, Archaeology and Chemistry, University of York, Heslington, York YO10 5DD, UK fTigh-na-Cleirich, Foss, nr Pitlochry, Perthshire PH16 5NQ, UK Received 14 June 2007; received in revised form 2 January 2008; accepted 4 January 2008 Abstract The climatic signal of Marine Isotope Stage (MIS) 11 is well-documented in marine and ice-sheet isotopic records and is known to comprise at least two major warm episodes with an intervening cool phase. Terrestrial records of MIS 11, though of high resolution, are often fragmentary and their chronology is poorly constrained. However, some notable exceptions include sequences from the maar lakes in France and Tenaghi Philippon in Greece. In the UK, the Hoxnian Interglacial has been considered to correlate with MIS 11. New investigations at Hoxne (Suffolk) provide an opportunity to re-evaluate the terrestrial record of MIS 11. At Hoxne, the type Hoxnian Interglacial sediments are overlain by a post-Hoxnian cold-temperate sequence. The interglacial sediments and the later temperate phase are separated by the so-called ‘Arctic Bed’ from which cold-climate macroscopic plant and beetle remains have been recovered. -

JOHN WYMER Copyright © British Academy 2007 – All Rights Reserved
JOHN WYMER Jim Rose Copyright © British Academy 2007 – all rights reserved John James Wymer 1928–2006 ON A WET JUNE DAY IN 1997 a party of archaeologists met at the Swan in the small Suffolk village of Hoxne to celebrate a short letter that changed the way we understand our origins. Two hundred years before, the Suffolk landowner John Frere had written to the Society of Antiquaries of London about flint ‘weapons’ that had been dug up in the local brickyard. He noted the depth of the strata in which they lay alongside the bones of unknown animals of enormous size. He concluded with great prescience that ‘the situation in which these weapons were found may tempt us to refer them to a very remote period indeed; even beyond that of the present world’.1 Frere’s letter is now recognised as the starting point for Palaeolithic, old stone age, archaeology. In two short pages he identified stone tools as objects of curiosity in their own right. But he also reasoned that because of their geological position they were ‘fabricated and used by a people who had not the use of metals’.2 The bi-centenary gathering was organised by John Wymer who devoted his professional life to the study of the Palaeolithic and whose importance to the subject extended far beyond a brickpit in Suffolk. Wymer was the greatest field naturalist of the Palaeolithic. He had acute gifts of observation and an attention to detail for both artefacts and geol- ogy that was unsurpassed. He provided a typology and a chronology for the earliest artefacts of Britain and used these same skills to establish 1 R. -

Position of the Steinheim Interglacial Sequence Within the Marine Oxygen Isotope Record Based on Mammal Biostratigraphy
Quaternary International 292 (2013) 33e42 Contents lists available at SciVerse ScienceDirect Quaternary International journal homepage: www.elsevier.com/locate/quaint Position of the Steinheim interglacial sequence within the marine oxygen isotope record based on mammal biostratigraphy Eline Naomi van Asperen*,1 Department of Archaeology, University of York, King’s Manor, York YO1 7EP, United Kingdom article info abstract Article history: Since the recovery, in 1933, of a Homo heidelbergensis skull from the interglacial Antiquus-Schotter in Available online 24 October 2012 sediments of the River Murr, the site of Steinheim (Germany) has been regarded as an iconic hominin locality for the Middle Pleistocene of Europe. Based on the morphology of the specimen, stratigraphical considerations and the characteristics of the associated faunal assemblage, the Steinheim skull has generally been assigned to the Holsteinian Interglacial. Over the last decades, developments in the knowledge of the complexity of the Pleistocene glacialeinterglacial cycles have rendered this date increasingly problematic. Analyses of caballoid horse remains from the site using log ratio diagrams and principal components analysis reveal striking morphological differences with German horse remains from sites attributed to the Holsteinian or marine isotope stages (MIS) 11 and 9. The morphology of the Steinheim horses is similar to that of horse specimens from MIS 9 sites in southern France, and to a lesser degree there are similarities with MIS 9 horses from northern France and British MIS 11 and 9 horse fossils. This indicates that during the Anglian/Elsterian glacial, European horse populations were split into two lineages. During MIS 11, the western lineage is present in the British Isles and at Steinheim, whereas the German Hol- steinian samples belong to the eastern lineage. -

Athelington (Otherwise Allington)
1. Parish: Athelington (otherwise Allington) Meaning: a. The homestead/village of the aethelings or princes b. The homestead/village of Aepelheah’s people 2. Hundred: Hoxne Deanery: Hoxne Union: Hoxne (1835–1907), Hartismere RD (1934–1974), Mid Suffolk DC (1974–) RDC/UDC: (E. Suffolk) Hoxne RD (1894–1934), Hartismere RD (1934–1974), Mid Suffolk DC (1974–) Other administrative details: Hoxne Petty Sessional Division Framlingham and Saxmundham County Court District 3. Area: 494 acres (1912) 4. Soils: slowly permeable seasonally waterlogged fine loam over clay 5. Types of farming: 1500–1640 Thirsk: Wood-pasture region, mainly pasture, meadow, engaged in rearing and dairying with some pig keeping, horse breeding and poultry 1818 Marshall: Course of crops varies usually including summer fallow as preparation for corn products 1937 Main crops: Wheat, barley, peas and beans 1969 Trist: More intensive cereal growing and sugar beet 6. Enclosure: 7. Settlement: 1958 Railway crossed parish from N–S Extremely small development around the church. Settlement positioned close to northern boundary Few scattered farms Inhabited houses: 1674 – 7, 1801 – 11, 1851 – 25, 1871 – 26, 1901 – 19, 1951 – 12, 1981 – 14 1 8. Communications: Road: To Bedingfield, Horham, Wilby and Worlingworth 1891 Carrier to Ipswich on Thursday Rail: 1891 4½ miles Eye Station: Mellis–Eye line opened 1865, closed for passengers 1931, closed for goods 1964 9. Population: 1086 – Not recorded 1327 – 32 taxpayers paid £3 11s. (includes Horham) 1524 – 8 taxpayers paid 19s. 2d. 1603 – 35 adults 1674 – 7 households 1676 – Not recorded 1801 – 70 inhabitants 1831 – 129 inhabitants 1851 – 117 inhabitants 1871 – 129 inhabitants 1901 – 87 inhabitants 1931 – 71 inhabitants 1951 – 38 inhabitants 1971 – 51 inhabitants 1981 – 35 inhabitants 10. -

Hoxne Conservation Area Appraisal
HOXNE CONSERVATION AREA APPRAISAL Hoxne: Low Street © Crown copyright All Rights Reserved M S D C Licence no 100017810 2010 INTRODUCTION The conservation area at Low Street, Hoxne was originally designated by East Suffolk County Council in 1973 and inherited by the newly formed Mid Suffolk District Council at its inception in 1974. The conservation area was last appraised and extended by Mid Suffolk District Council in 2000 to include an area at Cross Street. The Council has a duty to review its conservation area designations from time to time, and this appraisal examines Hoxne under a number of different headings as set out in English Heritage’s ‘Guidance on Conservation Area Appraisals’ (2006). As such it is a straightforward appraisal of Hoxne’s built environment in conservation terms. This document is neither prescriptive nor overly descriptive, but more a Banham Bricks, Cross Street demonstration of ‘quality of place’, sufficient for the briefing of the Planning Officer when assessing proposed works in the area. The photographs and maps are thus intended to contribute as much as the text itself. As the English Heritage guidelines point out, the appraisal is to be read as a general overview, rather than as a comprehensive listing, and the omission of any particular building, feature or space does not imply that it is of no interest in conservation terms. Text, photographs and map overlays by Patrick Taylor, Conservation Architect, Mid Suffolk District Council 2012. Village Green, Low Street Hoxne: Cross Street © Crown copyright All Rights Reserved M S D C Licence no 100017810 2010 TOPOGRAPHICAL FRAMEWORK The village of Hoxne consists of two main settlements: to the north Low Street and to the south Cross Street. -

A Memorial Tablet to John Frere, F.R.S., F.S.A
A MEMORIAL TABLET TO JOHN FRERE, F.R.S., F.S.A. A NOTE IN the 1998issueof the Proceedings (Vol.XXX1XPart 2, 268-69) gavea brief account of a visit made to Hoxne and Finningham on 22 June 1997 in order to commemorate the bicentenaryof the reading ofJohn Frere's letter to the SocietyofAntiquariesof London. This letter put forward incontrovertibleevidencefor the immenseantiquity of mankind, greatly at variancewith the accepted viewsof the time. The visitwasmade by a party of archaeologists whohad specialinterestsin the Palaeolithicperiod or the archaeologyof EastAnglia.The final callwasat Frere's burial place,St Bartholomew'sChurch, Finningham.The lackof any specific memorialtoJohn Frere, either here or elsewhere,wasdeplored. The President,who waswith the party,agreed with the company that the placingof a suitabletablet in the church wouldbe a fitting tribute to him. It washoped that the Diocesewould sanction this if money could be raised to produce one. If so, it would be placed in the chancel with other memorialsof the Frere family. It can now be happily reported tbat permissionwasforthcomingfrom the Dioceseand the • 4 A FIG. 162 —The John Frere memorial tablet in Finningham Church, Suffolk. Welsh slate with letters painted off-whiteand a replica flint hand-axe; designed and cut by the Cardozo-KindersleyWorkshop in Cambridge. 541 BUSINESS AND ACTIVITIES Parochial Church Council, and the tablet (Fig. 162) is on the wall of the chancel. It has been beautifully executed by the prestigious Cardozo-Kindersley Workshop in Cambridge, and includes a replica made by Phil Harding of one of the flint hand-axes as published by Frere in his letter to the Society of Antiquaries. -
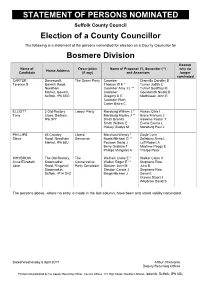
Statement of Persons Nominated
STATEMENT OF PERSONS NOMINATED Suffolk County Council Election of a County Councillor The following is a statement of the persons nominated for election as a County Councillor for Bosmere Division Reason Name of Description Name of Proposer (*), Seconder (**) why no Home Address Candidate (if any) and Assentors longer nominated CARTER Danescroft, The Green Party Coomber Granville Dorothy B Terence S Ipswich Road, Thomas W F * Turner Judith C Needham Coomber Amy J L ** Turner Geoffrey M Market, Ipswich, Coomber Gouldsmith Nicola B Suffolk, IP6 8EG Gregory D E Matthissen John E Coomber Ruth Carter Bistra C ELLIOTT 3 Old Rectory Labour Party Marsburg William J * Hiskey Clive I Tony Close, Barham, Marsburg Hayley J ** Brace Frances J IP6 0PY Smith Brenda Hawkins Kester T Smith William E Evans Emma L Hiskey Gladys M Marsburg Paul J PHILLIPS 46 Crowley Liberal Marchant Wendy * Gayle Lynn Steve Road, Needham Democrat Norris Michael G ** Salisbury Anna L Market, IP6 8BJ Poulson David J Luff Robert A Berry Graham T Mayhew Peggy E Phillips Margaret A Thorpe Peter WHYBROW The Old Rectory, The Welham Claire E * Walker Claire V Anne Elizabeth Stowmarket Conservative Walker Roger E ** Stephens-Row Jane Road, Ringshall, Party Candidate Stratton John M Julia B Stowmarket, Stratton Carole J Stephens-Row Suffolk, IP14 2HZ Brega Michael J David E Groves Stuart J Whybrow David S The persons above, where no entry is made in the last column, have been and stand validly nominated. Dated Wednesday 5 April 2017 Arthur Charvonia Deputy Returning Officer Printed