Evaluation of the Blood-Brain Barrier Permeability of 13 Related
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recommended Methods for the Identification and Analysis of Synthetic Cathinones in Seized Materialsd
Recommended methods for the Identification and Analysis of Synthetic Cathinones in Seized Materials (Revised and updated) MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Photo credits:UNODC Photo Library; UNODC/Ioulia Kondratovitch; Alessandro Scotti. Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Synthetic Cathinones in Seized Materials (Revised and updated) MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2020 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/49/REV.1 Original language: English © United Nations, March 2020. All rights reserved, worldwide. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. -

Issue 11 - Jan - Mar 2017 �� Collection Andtestingofsubstancesand,Most WEDINOS Quarterly Newsletter
Collecting, Testing, Informing wedinos.org BULLETIN WEDINOS Quarterly Newsletter Issue 11 - Jan Mar 2017 The WEDINOS project has been designed for the collection and testing of substances and, most importantly, dissemination via www.wedinos.org of pragmatic evidence based harm reduction WEDINOS information for users. WEDINOS aims to go beyond identification of novel substances, to address the Headlines harms associated with use of New Psychoactive Substances (NPS), new combinations of established drugs and NPS and Steroids & Image Enhancing Drugs (SIEDs). TOTAL number of samles received by WEDINOS October 201 to March 2017 This uarter ,2 (January 2017 to March 2017) Samles received 396 Samles received ,0 Samles 221 analysed Samles analysed 89 86 Samles Samles 7 ending 345 ending Substances Substances identified in identified in either either 89 combination or combination Samles isolation or isolation reected 1 FINDINGS… WHERE... Samples were submitted from six of the Breakdown of sample submissions by Health Board areas seven Welsh Health Boards. No samples 49 samples were received from England, were received form Powys Teaching A - Betsi Cadwaladr University Health five from Scotland and one from outside Health Board. Board – 20 samples. the United Kingdom. B - Powys Teaching Health Board – 0 samples. WEDINOS does not analyse samples C - Aneurin Bevan University Health A received from outside of the United Board – 62 samples. Kingdom. D - Cwm Taf University Health Board – 10 samples. In relation to Welsh Health Board areas, E - Cardiff & Vale University Health Board the highest proportion of samples came – 44 samples. from Aneurin Bevan University Health F - Abertawe Bro Morgannwg University B Board, 62 samples were received and Health Board – 21 samples. -

TURKEY MINISTRY of INTERIOR TURKISH NATIONAL POLICE Anti-Smuggling and Organized Crime Department
REPUBLIC OF TURKEY MINISTRY OF INTERIOR TURKISH NATIONAL POLICE Anti-Smuggling and Organized Crime Department 2014 NATIONAL REPORT (2013 data) TO THE EMCDDA by the Reitox National Focal Point TURKEY New Development, Trends and in-depth information on selected issues TURKISH MONITORING CENTRE FOR DRUGS AND DRUG ADDICTION (TUBİM) REITOX 1 TABLE OF CONTENTS TABLE OF CONTENTS ........................................................................................................... 2 TURKISH NATIONAL FOCAL POINT STAFF PREPARING THE REPORT ........................... 7 DATA PROVIDING AGENCIES AND AGENCY REPRESENTATIVES ................................... 8 PREFACE .............................................................................................................................. 11 ABBREVIATIONS .................................................................................................................. 13 SUMMARY ............................................................................................................................. 15 NEW DEVELOPMENTS AND TRENDS ................................................................................ 29 SECTION 1 ............................................................................................................................ 29 DRUG POLICY: LAWS, STRATEGIES, AND ECONOMIC ANALYSES ............................... 29 1.1. Introduction ................................................................................................................ 29 1.2. Legal Framework -

Model Scheduling New/Novel Psychoactive Substances Act (Third Edition)
Model Scheduling New/Novel Psychoactive Substances Act (Third Edition) July 1, 2019. This project was supported by Grant No. G1799ONDCP03A, awarded by the Office of National Drug Control Policy. Points of view or opinions in this document are those of the author and do not necessarily represent the official position or policies of the Office of National Drug Control Policy or the United States Government. © 2019 NATIONAL ALLIANCE FOR MODEL STATE DRUG LAWS. This document may be reproduced for non-commercial purposes with full attribution to the National Alliance for Model State Drug Laws. Please contact NAMSDL at [email protected] or (703) 229-4954 with any questions about the Model Language. This document is intended for educational purposes only and does not constitute legal advice or opinion. Headquarters Office: NATIONAL ALLIANCE FOR MODEL STATE DRUG 1 LAWS, 1335 North Front Street, First Floor, Harrisburg, PA, 17102-2629. Model Scheduling New/Novel Psychoactive Substances Act (Third Edition)1 Table of Contents 3 Policy Statement and Background 5 Highlights 6 Section I – Short Title 6 Section II – Purpose 6 Section III – Synthetic Cannabinoids 13 Section IV – Substituted Cathinones 19 Section V – Substituted Phenethylamines 23 Section VI – N-benzyl Phenethylamine Compounds 25 Section VII – Substituted Tryptamines 28 Section VIII – Substituted Phenylcyclohexylamines 30 Section IX – Fentanyl Derivatives 39 Section X – Unclassified NPS 43 Appendix 1 Second edition published in September 2018; first edition published in 2014. Content in red bold first added in third edition. © 2019 NATIONAL ALLIANCE FOR MODEL STATE DRUG LAWS. This document may be reproduced for non-commercial purposes with full attribution to the National Alliance for Model State Drug Laws. -

Buy Quality Mdma , Mdpv , Methylone , 4Mmc . 2Ci , 2Ce , Cocaine , Mephedrone Health & Beauty ,France
AKClassy.com Buy quality mdma , mdpv , methylone , 4mmc . 2ci , 2ce , cocaine , mephedrone Health & Beauty ,France DESCRIPTION https://deltabioresearchchemicals.com We supply good quality Research Chemicals,VENOMOUS, Benzodiazepines, Pain Medications, Anxiety Medications, Relief Medications, Insomnia Medications,VENOMOUS,Weight Lost Medications, Stimulants, Opiate/Opioid, Research Chemicals&VENOMOUS, etc... How to Order Prescription Drugs Safely Online | Where to Order Prescription Drugs Safely Online. https://deltabioresearchchemicals.com skype: live:deltabioresearchchemicals Text & Call:+86 (184) 7507-2355 Text:+1(619) 356-8501 Whatsapp:+1(619) 356-8501 Email:[email protected] Buy at good and affordable prices: ==> Research Chemicals, ==> Benzodiazepines, ==> Stimulants, ==> Opiate/Opioid, ==> Pain pills, ==> Anxiety pills, ==> Relief pills, ==> Insomnia pills, ==> Weight Lost pills, ==> Medical Marijuana, ==> Steroids, ==> HGH, ==> VENOMOUS ..etc... Products Available In Stock: venom: snake Venoms Spiders Venoms Scorpions Venoms more *** SPECIAL SALES *** Buy Liquid Mercury, Buy Potassium Cyanide (KCN), Buy GHB, Buy Ketamine, Buy Nembutal, Buy Seconal, Buy LSD, Buy Ecstasy, ==>> CANNABINOIDS: Buy 4-CN-BINACA-ADB, Buy 5-CL-ADB-A, Buy 5F-AB-PINACA, Buy AB-001, Buy AB-CHMINACA, Buy AKB-57, Buy AMB-FUBINACA, Buy Anandamide, Buy CUMYL-4CN-BINACA, Buy CUMYL-PINACA, Buy FUB-PB-22, Buy MA-CHMINACA, Buy MDMB-CHMINACA, _more =>> Buy STIMULANTS ONLINE: -Buy 5-MAPB, Buy 5-Methylethylone, Buy Carfentanil, Buy Dibutylone, Buy -

Issue 12 - Apr - June 2017 Identified in Substances Collection Andtestingofsubstancesand,Most Isolation
Collecting, Testing, Informing wedinos.org BULLETIN WEDINOS Quarterly Newsletter Issue 12 - Apr - June 2017 Issue 12 - The WEDINOS project has been designed for the collection and testing of substances and, most importantly, dissemination via www.wedinos.org of WEDINOS pragmatic evidence based harm reduction information Headlines for users. WEDINOS aims to go beyond identification of novel substances, to address the harms associated with use of New Psychoactive Substances (NPS), new TOTAL number of samples receied combinations of established drugs and NPS and Image by WEDINOS October 201 to arch 2017 and Performance Enhancing Drugs (IPEDs). For more information on IPEDs please visit: This uarter www.ipedinfo.co.uk. ,7 April 2017 to June th 2017 Samples receied 22 Please note that figures for this quarterly Samples report include samples submitted and receied analysed up to and including 9th June ,0 2017. This was due to a combination of Samples 17 essential maintenance to the WEDINOS analysed Samples analytical tools and a review of the analysed programme risk assessment. 1,00 21 Samples Samples 53 reected 7 reected Substances Substances identified in identified in either either combination or combination isolation or isolation Fie synthetic cannabinoid receptor agonists Fie designer benodiaepines Fentanyl / Fentanyl type-substances / Synthetic Opioids Carfentanyl and mannitol Heroin, noscapine and Thiofentanyl Acetylfentanyl Ocfentanil, paracetamol furanylfentanyl and caffeine Due to recent fatalities in North England and media attention Fentanyl and Fentanyl type-substances are very topical at this time. Synthetic opioids are man-made substances that have been manufactured to mimic the effects of natural opioids including opium. This category of substances includes prescription only medicines (POMs) including fentanyl and pethidine (fentanyl and pethidine are also controlled by the Misuse of Drugs Act 1971 as a Class A substance), and NPS including AH-7291 and MT-45 which have very little history of use and no evidenced medicinal uses. -
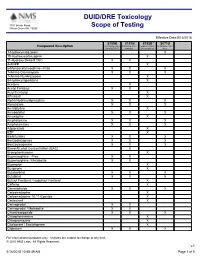
DUID/DRE Toxicology Scope of Testing
DUID/DRE Toxicology 3701 Welsh Road Willow Grove PA, 19090 Scope of Testing Effective Date:5/16/2018 8150B 8151B 8152B* 8071U Compound Description DUID/DRE Panel DUID/DRE Panel Expanded Drug DUID/DRE Panel, ProofPOSITIVE w/alcohol Screen Add-on Urine 1-Hydroxymidazolam X 10-Hydroxycarbazepine X 11-Hydroxy Delta-9 THC X X 4-ANPP X 6-Monoacetylmorphine - Free X X X 7-Amino Clonazepam X X X 7-Amino Flunitrazepam X 9-Hydroxyrisperidone X Acetone X Acetyl Fentanyl X X Acryl Fentanyl X Alfentanil X Alpha-Hydroxyalprazolam X X X Alprazolam X X X Amitriptyline X Amobarbital X X X Amoxapine X Amphetamine X X X Amphetamines X X X Aripiprazole X BZP X Barbiturates X X X Benzodiazepines X X X Benzoylecgonine X X X Blood Alcohol Concentration (BAC) X Brompheniramine X Buprenorphine - Free X X Buprenorphine / Metabolite X X Bupropion X Buspirone X Butabarbital X X X Butalbital X X X Butyryl Fentanyl / Isobutyryl Fentanyl X Caffeine X Cannabinoids X X X Carbamazepine X Carbamazepine-10,11-Epoxide X Carfentanil X Carisoprodol X X Carisoprodol / Metabolite X X Chlordiazepoxide X X X Chlorpheniramine X Chlorpromazine X Citalopram / Escitalopram X Clobazam X X X For informational purposes only. Analytes are subject to change at any time. © 2010 NMS Labs. All Rights Reserved. v.1 5/16/2018 10:58:49 AM Page 1 of 5 8150B 8151B 8152B* 8071U Compound Description DUID/DRE Panel DUID/DRE Panel Expanded Drug DUID/DRE Panel, ProofPOSITIVE w/alcohol Screen Add-on Urine Clomipramine X Clonazepam X X Clonazolam X Clonidine X Clozapine X Cocaethylene X X X Cocaine X -

Fatal Intoxication with U-47700 in Combination with Other NPS (N
Forensic Toxicology https://doi.org/10.1007/s11419-020-00568-1 CASE REPORT Fatal intoxication with U‑47700 in combination with other NPS (N‑ethylhexedrone, adinazolam, 4‑CIC, 4‑CMC) confrmed by identifcation and quantifcation in autopsy specimens and evidences Karolina Nowak1,2 · Paweł Szpot1,2 · Marcin Zawadzki1,2 Received: 12 September 2020 / Accepted: 22 December 2020 © The Author(s) 2021 Abstract Purpose We present a case of fatal intoxication with U-47700 in combination with other NPS (N-ethylhexedrone, adinazolam, 4-chloro-N-isopropylcathinone (4-CIC), 4-chloromethcathinone (4-CMC) and sertraline) confrmed by identifcation and quantifcation in biological materials and evidences found at the scene in 2017 in Poland. Methods Blood and urine samples were extracted with ethyl acetate from alkaline medium (pH 9); powders/crystals were diluted with methanol. The analysis was carried out using ultra-high-performance liquid chromatography–tandem mass spectrometry. Validation criteria were evaluated for blood and urine at the concentrations of 10 and 100 ng/mL. Results The validation parameters of the method were within acceptable ranges. In the presented case, the determined concentrations of drugs were as follows, in blood: U-47700, 1470 ng/mL; N-ethylhexedrone, 58 ng/mL; adinazolam, 18 ng/ mL; 4-CIC, 8.0 ng/mL; 4-CMC, 1.7 ng/mL; in urine: U-47700, 3940 ng/mL; N-ethylhexedrone, 147 ng/mL; adinazolam, 82 ng/mL; 4-CIC, 130 ng/mL; 4-CMC, 417 ng/mL. Sertraline (blood, 89 ng/mL; urine, 32 ng/mL) was also determined in both materials. The same substances were also found in 5 powders/crystals: U-47700 (12% by weight), N-ethylhexedrone (54%), adinazolam (14%), 4-CIC (23%), 4-CMC (26%). -
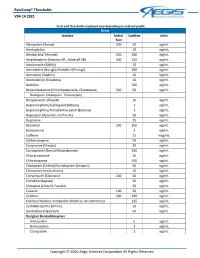
Paincomp® Thresholds V04.14.2021 Copyright © 2020 Aegis Sciences
PainComp® Thresholds V04.14.2021 Tests and thresholds employed vary depending on ordered profile Urine Analyte Initial Confirm Units Test Alprazolam (Xanax) 200 50 ng/mL Amitriptyline 10 ng/mL Amobarbital (Amytal) 200 200 ng/mL Amphetamine (Adzenys ER , Adderall XR) 500 250 ng/mL Aripiprazole (Abilify) 10 ng/mL Armodafinil (Nuvigil)/Modafinil (Provigil) 100 ng/mL Asenapine (Saphris) 10 ng/mL Atomoxetine (Strattera) 10 ng/mL Baclofen 500 ng/mL Benzodiazepines (Chlordiazepoxide, Clorazepate, 200 50 ng/mL Diazepam, Oxazepam, Temazepam) Brexpiprazole (Rexulti) 10 ng/mL Buprenorphine/Sublingual (Belbuca) 1 ng/mL Buprenorphine/Transdermal patch (Butrans) 1 ng/mL Bupropion (Aplenzin, Forfivo XL) 50 ng/mL Buspirone 25 ng/mL Butalbital 200 200 ng/mL Butorphanol 1 ng/mL Caffeine 15 mcg/mL Carbamazepine 50 ng/mL Cariprazine (Vraylar) 20 ng/mL Carisoprodol (Soma)/Meprobamate 200 ng/mL Chlorpromazine 10 ng/mL Chlorzoxazone 500 ng/mL Citalopram (Celexa)/Escitalopram (Lexapro) 50 ng/mL Clomipramine (Anafranil) 10 ng/mL Clonazepam (Klonopin) 200 50 ng/mL Clonidine (Kapvay) 50 ng/mL Clozapine (Clozaril, Fazaclo) 50 ng/mL Cocaine 100 50 ng/mL Codeine 200 100 ng/mL Cotinine/Nicotine metabolite (Habitrol, Nicoderm CQ) 125 ng/mL Cyclobenzaprine (Amrix) 10 ng/mL Dantrolene (Dantrium) 50 ng/mL Designer Benzodiazepines Adinazolam 5 ng/mL Bromazolam 1 ng/mL Clonazolam 1 ng/mL Copyright © 2020 Aegis Sciences Corporation All Rights Reserved PainComp® Thresholds V04.14.2021 Deschloroetizolam 1 ng/mL Diclazepam 1 ng/mL Etizolam 1 ng/mL Flualprazolam 1 ng/mL Flubromazepam -

The Newest Cathinone Derivatives As Designer Drugs: an Analytical and Toxicological Review
Forensic Toxicol DOI 10.1007/s11419-017-0385-6 REVIEW ARTICLE The newest cathinone derivatives as designer drugs: an analytical and toxicological review 1,3 3 2 1 Milena Majchrzak • Rafał Celin´ski • Piotr Kus´ • Teresa Kowalska • Mieczysław Sajewicz1 Received: 30 July 2017 / Accepted: 22 August 2017 Ó The Author(s) 2017. This article is an open access publication Abstract Conclusions To our knowledge, this is the most current Purpose Currently, among new psychoactive substances, review presenting new synthetic cathinones. Political cathinone derivatives constitute the biggest group, which authorities should take measures to implement and enforce are mainly classified into N-alkylated, 3,4-methylenedioxy- generic scheduling (comprehensive system) laws to control N-alkylated, N-pyrrolidinyl, and 3,4-methylenedioxy-N- the diversely modified synthetic cathinones. Supplement- pyrrolidinyl derivatives. These derivatives are actively ing the existing databases with new findings can greatly being subjected to minor modifications at the alkyl chains facilitate the efforts of forensic toxicologists. or the aromatic ring to create new synthetic cathinones with the goal of circumventing laws. In this review, the new Keywords New synthetic cathinones Á Designer drugs Á synthetic cathinones that have appeared on the illegal drug NPS Á LC–MS Á GC–MS Á NMR market during the period 2014–2017 are highlighted, and their characterization by gas chromatography–mass spec- trometry and liquid chromatography–tandem mass spec- Introduction trometry is presented. Methods Various key words were used to conduct an Cathinone is one of the biologically active alkaloids found extensive literature search across a number of databases, in the khat shrub (Catha edulis). -

Bf2f8ee7-3380-4536-Adc9
THE STATUTES OF THE REPUBLIC OF SINGAPORE MISUSE OF DRUGS ACT (CHAPTER 185) (Original Enactment: Act 5 of 1973) REVISED EDITION 2008 (31st March 2008) Prepared and Published by THE LAW REVISION COMMISSION UNDER THE AUTHORITY OF THE REVISED EDITION OF THE LAWS ACT (CHAPTER 275) Informal Consolidation – version in force from 1/5/2021 CHAPTER 185 2008 Ed. Misuse of Drugs Act ARRANGEMENT OF SECTIONS PART I PRELIMINARY Section 1. Short title 2. Interpretation 3. Appointment of Director and other officers of Central Narcotics Bureau 4. Advisory committees PART II OFFENCES INVOLVING CONTROLLED DRUGS AND SUBSTANCES 5. Trafficking in controlled drugs 6. Manufacture of controlled drugs 7. Import and export of controlled drugs 8. Possession and consumption of controlled drugs 8A. Consumption of drug outside Singapore by citizen or permanent resident 9. Possession of pipes, utensils, etc. 10. Cultivation of cannabis, opium and coca plants 10A. Manufacture, supply, possession, import or export of equipment, materials or substances useful for manufacture of controlled drugs 10B. Regulations on controlled equipment, material or substances 11. Responsibilities of owners, tenants, etc. 11A. Arranging or planning gatherings where controlled drugs are to be consumed or trafficked 11B. Exposing child to drugs, etc., and permitting young person to consume drugs 11C. Introducing drug trafficker to another person 11D. Instructing person to cultivate cannabis, etc., or to manufacture or consume controlled drugs, etc. 11E. Causing or procuring young person or vulnerable person to commit certain offences 1 Informal Consolidation – version in force from 1/5/2021 2008 Ed. Misuse of Drugs CAP. 185 2 Section 12. -

Critical Review Report: N-Ethylhexedrone
Critical Review Report: N-Ethylhexedrone Expert Committee on Drug Dependence Forty-second Meeting Geneva, 21-25 October 2019 This report contains the views of an international group of experts, and does not necessarily represent the decisions or the stated policy of the World Health Organization 42nd ECDD (2019): N-ethylhexedrone © World Health Organization 2019 All rights reserved. This is an advance copy distributed to the participants of the 42nd Expert Committee on Drug Dependence, before it has been formally published by the World Health Organization. The document may not be reviewed, abstracted, quoted, reproduced, transmitted, distributed, translated or adapted, in part or in whole, in any form or by any means without the permission of the World Health Organization. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.