Modern Approaches to the Synthesis and Transformations of Practically Valuable Benzothiazole Derivatives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthesis of Condensed 1,2,4-Triazolo-Heterocycles
Chemistry SYNTHESIS OF CONDENSED 1,2,4-TRIAZOLO-HETEROCYCLES MOHAMMED A. E. SHABAN ADEL Z. NASR MAMDOUH A. M. TAHA SUMMARY: Cyclization of 2-hydrazino-1, 3-benzothiazole, 2-hydrozinoquioline, 2-hydrazinolepidine, and 2-hydrazino-pyridine with one-carbon cyclizing agents such as triethyl orthoformate, ethyl chlorofor- mate, urea, phenylthiourea, and carbon disulfide gave 3-substituted-1,2,4-triazolo (3,4, -b) 1, 3-benzothia- zoles, 3-substituted-1,2,4-triazolo (4,3-a) quinolines, 3-substituted-1,2,4-triazolo (4,3-a) quinolines, 3-substituted-1,2,4-triazolo (4,3-a) lepidines and 3-substituted-1,2,4-triazolo (4,3-a) pyridines respectively. Reactions with acetic acid and acetic anhydride gave the corresponding acetyl hydrazines which were cyclized to the 3-methyl 1,2,4-triazolo-heterocyles. Ring closure with phenyl isocyanate and phenyl isothio- cyanate, gave the intermediate 4-phenylsemicarbazides and 4-phenylthiosemicarbazides which, upon fusion, afforded the corresponding 3-oxo- and 3-thioxo-1,2,4-triazolo-heterocyles. The 3-oxo-compounds were also obtained when 2-chloroquinoloni or 2-chlorolepidine was fused with semicarbazide hydrochloride. Key Words: Synthesis, amidrazones, triazolo-heterocycles. INTRODUCTION The synthesis and biological activities of condensed In an attempt to prepare 3-methyl-1,2,4-triazolo (3,4-b) 1,2,4-triazolo (3,4-z) heterocyles have recently been 1,3-benzothiazole (5a) by heating 2-hydrazino-1, 3- reviewed (14). Several condendes 1,2,4-triazolo-hetero- benzothiazole (1a) with an excess of acetic acid or acetic cyles exhibit various biological activities such as fungicidal anhydride, 1,1,2-triacetyl-2-(1,3-benzothiazol-2-yl) hyd- (1,9) bactericidal (1,9) analgesic (4,7,10) anxiolytic (8) razine (3a) was obtained. -
![Synthesis of [1,3,2]Dithiazolo[4,5-B][1,2,5]Oxadiazolo[3,4-E]Pyrazines](https://docslib.b-cdn.net/cover/0279/synthesis-of-1-3-2-dithiazolo-4-5-b-1-2-5-oxadiazolo-3-4-e-pyrazines-270279.webp)
Synthesis of [1,3,2]Dithiazolo[4,5-B][1,2,5]Oxadiazolo[3,4-E]Pyrazines
General Papers ARKIVOC 2011 (xi) 69-81 Synthesis of [1,3,2]dithiazolo[4,5-b][1,2,5]oxadiazolo[3,4-e]pyrazines Lidia S. Konstantinova,a Vadim V. Popov,a Natalia V. Obruchnikova,a Konstantin A. Lyssenko,b Ivan V. Ananyev,b and Oleg A. Rakitina* aN. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninsky Prospect, 47, 119991 Moscow, Russia bA. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilov Str., 28, 119991 Moscow, Russia E-mail: [email protected] Abstract The reaction temperature has a strong impact on the results of chlorination of 5,6-bis(tert- butylthio)[1,2,5]oxadiazolo[3,4-b]pyrazine that is readily prepared from 5,6- dichloro[1,2,5]oxadiazolo[3,4-b]pyrazine and sodium tert-butylsulfide. Mono- and bis(sulfenylchlorides) were selectively obtained in high yield and their structure was confirmed by the reaction with morpholine. Treatment of [1,2,5]oxadiazolo[3,4-b]pyrazine-5,6-disulfenyl dichloride with primary aliphatic amines and benzylamine afforded N-substituted [1,3,2]dithiazolo[4,5-b][1,2,5]oxadiazolo[3,4-e]pyrazines in moderate yields. Novel pentacyclic [1,2,5]oxadiazolo[3'',4'':5',6']pyrazino[2',3':5,6][1,2,4]thiadiazino[3,4-b][1,3]benzothiazole, whose structure was confirmed by X-ray diffraction, was obtained by the reaction of this disulfenyl dichloride with 2-aminobenzothiazole. Keywords: Fused 1,3,2-dithiazoles, [1,2,5]oxadiazolo[3,4-b]pyrazines, disulfenyl dichlorides, primary amines, bis(tert-butylthio) derivatives, chlorination Introduction Amongst five-membered -
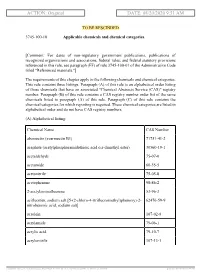
ACTION: Original DATE: 08/20/2020 9:51 AM
ACTION: Original DATE: 08/20/2020 9:51 AM TO BE RESCINDED 3745-100-10 Applicable chemicals and chemical categories. [Comment: For dates of non-regulatory government publications, publications of recognized organizations and associations, federal rules, and federal statutory provisions referenced in this rule, see paragraph (FF) of rule 3745-100-01 of the Administrative Code titled "Referenced materials."] The requirements of this chapter apply to the following chemicals and chemical categories. This rule contains three listings. Paragraph (A) of this rule is an alphabetical order listing of those chemicals that have an associated "Chemical Abstracts Service (CAS)" registry number. Paragraph (B) of this rule contains a CAS registry number order list of the same chemicals listed in paragraph (A) of this rule. Paragraph (C) of this rule contains the chemical categories for which reporting is required. These chemical categories are listed in alphabetical order and do not have CAS registry numbers. (A) Alphabetical listing: Chemical Name CAS Number abamectin (avermectin B1) 71751-41-2 acephate (acetylphosphoramidothioic acid o,s-dimethyl ester) 30560-19-1 acetaldehyde 75-07-0 acetamide 60-35-5 acetonitrile 75-05-8 acetophenone 98-86-2 2-acetylaminofluorene 53-96-3 acifluorfen, sodium salt [5-(2-chloro-4-(trifluoromethyl)phenoxy)-2- 62476-59-9 nitrobenzoic acid, sodium salt] acrolein 107-02-8 acrylamide 79-06-1 acrylic acid 79-10-7 acrylonitrile 107-13-1 [ stylesheet: rule.xsl 2.14, authoring tool: RAS XMetaL R2_0F1, (dv: 0, p: 185720, pa: -
![Pressure Synthesis of Anti-Cancer Active Thiazolo[4,5-C]](https://docslib.b-cdn.net/cover/6436/pressure-synthesis-of-anti-cancer-active-thiazolo-4-5-c-696436.webp)
Pressure Synthesis of Anti-Cancer Active Thiazolo[4,5-C]
www.nature.com/scientificreports There are amendments to this paper OPEN The frst Q-Tube based high- pressure synthesis of anti-cancer active thiazolo[4,5-c]pyridazines via the [4 + 2] cyclocondensation of 3-oxo-2-arylhydrazonopropanals with 4-thiazolidinones Hamada Mohamed Ibrahim 1,2 & Haider Behbehani 1 A novel and efcient protocol for the synthesis of thiazolo[4,5-c]pyridazine derivatives was developed. The approach utilizes a high pressure Q-Tube reactor to promote cyclocondensation reactions between 3-oxo-2-arylhydrazonopropanals and 4-thiazolidinones. The process has a signifcantly high atom economy and a broad substrate scope, as well as being applicable to gram scale syntheses. The in vitro cytotoxic activities of the synthesized thiazolo[4,5-c]pyridazine derivatives were examined utilizing a MTT colorimetric assay with doxorubicin as a reference anti-cancer drug and three human cancer cell lines including HCT-116 (colon), MCF-7 (breast) and A549 (lung). The results show that thiazolopyridazines 7c, h, k and p have high cytotoxic activity against the MCF-7 cell line with respective IC50 values of 14.34, 10.39, 15.43 and 13.60 μM. Moreover, the thiazolopyridazine derivative 7s also show promising cytotoxic activity against the HCT-116 cell line with IC50 = 6.90 μM . Observations made in this efort serve as a basis for further investigations into the design and preparation of new anti-cancer drugs. Tiazolopyridazine derivatives comprise a broad range of structurally interesting substances that display a variety of medicinally interesting properties including activities against cancers1,2, microbes3,4, viruses5 and bacteria6, as well as antioxidant7, analgesic and pesticidal activities8,9. -

Transforming Litmp Lithiation of Challenging Diazines Through Gallium Alkyl Trans-Metal-Trapping Marina Uzelac, Alan R
Angewandte Communications Chemie International Edition: DOI: 10.1002/anie.201607284 Metalation German Edition: DOI: 10.1002/ange.201607284 Transforming LiTMP Lithiation of Challenging Diazines through Gallium Alkyl Trans-Metal-Trapping Marina Uzelac, Alan R. Kennedy, Eva Hevia,* and Robert E. Mulvey* Abstract: This study establishes a new trans-metal-trapping in a 1:1 base:pyrazine stoichiometry.[8] Moreover, a lack of (TMT) procedure based on a mixture of LiTMP (the base) and definitive structural information still impoverishes under- tris(trimethylsilylmethyl)gallium [Ga(CH2SiMe3)3, GaR3] (the standing of this area, which in the most extreme “black box” trap) that, operating in a tandem manner, is effective for the cases leads to a misidentification of the actual metalating regioselective deprotonation of sensitive diazines in hydro- base.[9] carbon solution, as illustrated through reactions of pyrazine, This paper reports a new trans-metal-trapping (TMT) pyridazine, and pyrimidine, as well as through the N-S procedure based on a mixture of LiTMP and tris(trimethylsi- heterocycle benzothiazole. The metallo-activated complexes lylmethyl)gallium [Ga(CH2SiMe3)3, GaR3] that is effective for of all of these compounds were isolated and structurally regioselective diazine and benzothiazole deprotonation in defined. hydrocarbon solution. Whereas alkyllithium reactions are generally irreversible, LiTMP reactions tend to be pKa- As one of the most used strategies in synthetic campaigns, dependent equilibria. In TMT, these equilibria are shifted -

TR-332: 2-Mercaptobenzothiazole (CASRN 149-30-4) in F344/N Rats and B6c3f1mice (Gavage Studies)
NATIONAL TOXICOLOGY PROGRAM Technical Report Series No. 332 TOXICOLOGY AND CARCINOGENESIS STUDIES OF 2-MERCAPTOBENZOTHIAZOLE (CAS NO. 149-30-4) IN F344/N RATS AND B6C3F1 MICE (GAVAGE STUDIES) U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health NATIONAL TOXICOLOGY PROGRAM The National Toxicology Program (NTP), established in 1978,develops and evaluates scientific information about potentially toxic and hazardous chemicals. This knowledge can be used for protecting the health of the American people and for the primary prevention of disease. By bringing to- gether the relevant programs, staff, and resources from the US. Public Health Service, DHHS, the National Toxicology Program has centralized and strengthened activities relating to toxicology research, testing and test developmenthalidation efforts, and the dissemination of toxicological in- formation to the public and scientific communities and to the research and regulatory agencies. The NTP is made up of four charter DHHS agencies: the National Cancer Institute (NCI), National Institutes of Health; the National Institute of En- vironmental Health Sciences (NIEHS), National Institutes of Health; the National Center for Toxicological Research (NCTR), Food and Drug Ad- ministration; and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control. In July 1981, the Carcino- genesis Bioassay Testing Program, NCI, was transferred to the NIEHS. 2-Mercaptobenzothiazole,NTP TR 332 NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF 2-MERCAPTOBENZOTHIAZOLE (CAS NO. 149-30-4) IN F344/N RATS AND B6C3F1 MICE (GAVAGE STUDIES) Michael P. Dieter, Ph.D., Chemical Manager NATIONAL TOXICOLOGY PROGRAM P.O. Box S2233 Research Triangle Park, NC 27709 May 1988 NTP TR 332 NIH Publication No. -

Deprotonative Metalation of Five-Membered Aromatic Heterocycles Using Mixed Lithium-Zinc Species J.M
Deprotonative metalation of five-membered aromatic heterocycles using mixed lithium-zinc species J.M. l’Helgoual’Ch, Anne Seggio, Floris Chevallier, M. Yonehara, Erwann Jeanneau, Masanobu Uchiyama, Florence Mongin To cite this version: J.M. l’Helgoual’Ch, Anne Seggio, Floris Chevallier, M. Yonehara, Erwann Jeanneau, et al.. Deproto- native metalation of five-membered aromatic heterocycles using mixed lithium-zinc species. Journal of Organic Chemistry, American Chemical Society, 2007, 73 (1), pp.177-183. 10.1021/jo7020345. hal-00784514 HAL Id: hal-00784514 https://hal.archives-ouvertes.fr/hal-00784514 Submitted on 5 Feb 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Deprotonative metalation of five-membered aromatic heterocycles using mixed lithium-zinc species Jean-Martial L'Helgoual'ch,† Anne Seggio,† Floris Chevallier,† Mitsuhiro Yonehara,‡ Erwann Jeanneau,§ Masanobu Uchiyama,*,‡ and Florence Mongin*,† Synthèse et ElectroSynthèse Organiques, UMR 6510 CNRS, Université de Rennes 1, Bâtiment 10A, Case 1003, Campus Scientifique de Beaulieu, 35042 Rennes, France, The Institute of Physical and Chemical Research, RIKEN, 2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan, and Centre de Diffractométrie Henri Longchambon, Université Claude Bernard Lyon 1, 69622 Villeurbanne, France [email protected], [email protected] RECEIVED DATE (to be automatically inserted after your manuscript is accepted if required according to the journal that you are submitting your paper to) † UMR 6510 CNRS, Université de Rennes 1. -

Wednesday, July 10, 2019 4:00Pm
Wednesday, July 10, 2019 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Melissa Abbott, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – July 10, 2019 DATE: July 3, 2019 NOTE: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the July meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/SoonerPsych Program Update – Appendix B Action Item – Vote to Prior Authorize Jornay PM™ [Methylphenidate Extended-Release (ER) Capsule], Evekeo ODT™ [Amphetamine Orally Disintegrating Tablet (ODT)], Adhansia XR™ (Methylphenidate ER Capsule), and Sunosi™ (Solriamfetol Tablet) – Appendix C Action Item – Vote to Prior Authorize Balversa™ (Erdafitinib) – Appendix D Action Item – Vote to Prior Authorize Annovera™ (Segesterone Acetate/Ethinyl Estradiol Vaginal System), Bijuva™ (Estradiol/Progesterone Capsule), Cequa™ (Cyclosporine 0.09% Ophthalmic Solution), Corlanor® (Ivabradine Oral Solution), Crotan™ (Crotamiton 10% Lotion), Gloperba® (Colchicine Oral Solution), Glycate® (Glycopyrrolate Tablet), Khapzory™ (Levoleucovorin Injection), Qmiiz™ ODT [Meloxicam Orally Disintegrating Tablet (ODT)], Seconal Sodium™ (Secobarbital -

A Simple, Rapid and Efficient One-Pot Protocol for the Synthesis of 2-Substituted Benzothiazole Derivatives and Their Antimicrobial Screening
RESEARCH ARTICLE A. Rauf, S. Gangal, S. Sharma and M. Zahin, 63 S. Afr. J. Chem., 2008, 61, 63–67, <http://journals.sabinet.co.za/sajchem/>. A Simple, Rapid and Efficient One-pot Protocol for the Synthesis of 2-substituted Benzothiazole Derivatives and their Antimicrobial Screening Abdul Rauf,a* Saloni Gangal,a Shweta Sharmaa and Maryam Zahinb aDepartment of Chemistry, Aligarh Muslim University, Aligarh 2002002, India. bDepartment of Agricultural Microbiology, Aligarh Muslim University, Aligarh 2002002, India. Received 30 July 2007, revised 31 August 2007, accepted 14 May 2008. ABSTRACT A rapid and efficient condensation reaction of 2-aminothiophenol with various fatty acids in solvent-free conditions with or without microwave irradiation was carried out to afford the corresponding 2-substituted benzothiazole derivatives in good to excellent yields. The structures of the new products were established by elemental analyses, IR, 1H NMR, 13C NMR and mass spectral data. All the title compounds were screened for their antibacterial and antifungal activity. Most of the compounds exhibited good antimicrobial activity. KEYWORDS Fatty acids, 2-substituted benzothiazole, 2-aminothiophenol, antimicrobial activity, microwave irradiation. 1. Introduction in the presence of nitrobenzene/SiO2 , or nitrobenzene/montmo- 2-Substituted benzothiazoles are an important group of rillonite K10,18 or carboxylic acids.19 heterocyclic compounds that have widespread applications in To the best of our knowledge 2-substituted benzothiazole pharmaceutical and industrial research. The benzothiazoyl derivatives of fatty acids have not yet been reported. The present moiety is a structural element of compounds with potent and work is a continuation of our study on the derivatization of fatty selective antitumour activity,1 antiviral,2 anticonvulsant,3 neuro- acids. -

Page 1 of 8 CONSENT ORDER for ESTABLISHMENT AND
ANDHRA PRADESH POLLUTION CONTROL BOARD D.No. 33-26-14 D/2, Near Sunrise Hospital, Pushpa Hotel Centre, Chalamalavari Street, Kasturibaipet, Vijayawada 520010. Website :www.appcb.ap.nic.in CONSENT ORDER FOR ESTABLISHMENT AND OPERATION Order No. 135 /APPCB/CFE/RO-VSP/HO/2014 Dt: 26.04.2018 Sub: APPCB CFE - M/s. Torrent Pharmaceuticals Ltd., (Formerly M/s. Glochem Industries Ltd.,) Plot No: 77, JN Pharmacity, Thanam (V), Parawada (M), Visakhapatnam District Consent for Establishment (CFE) and Consent for Operation (CFO) for Change of Product Mix under Sec. 25 / 26 of Water (P & C of P) Act, 1974 and Under Sec. 21 of Air (P&C of P) Act, 1981 - Issued - Reg. Ref: 1) CFE expansion order dt. 10.06.2016. 2) received through A.P. Single Desk Portal on 27.03.2018. 3) 10.04.2018. 4) CFE Committee meeting held on 24.04.2018. 5) 25.04.2018. 1. In the reference 2nd cited, an application was submitted to the Board seeking Consent for Establishment (CFE) and Consent for Operation order (CFO) for Change of Product Mix within the existing premises to produce the products with installed capacities as mentioned below, with an additional investment of Rs. 0.34 Crore. As per CFE (expansion) order dt. 10.06.2016: S. Quantity Name of the Product No (kg/day) 1 RALOXIFINE HYDROCHLORIDE 16.66 2 AMLODIPINE BESILATE 27.77 3 CETIRIZINE DIHYDROCHLORIDE 1.389 4 CLOPIDOGREL BESILATE 2.22 5 RABEPRAZOLE SODIUM 4.166 6 OLANZAPINE 3.333 7 TRIENTINE DI HYDROCHLORIDE 0.277 8 ALFUZOSIN HYDROCHLORIDE 2.777 9 AMISULPRIDE 1.389 10 LEVOCETIRIZINE DIHYDROCHLORIDE 8.333 11 TERBINAFINE -

Imports of Benzenoid Chemicals and Products in 1977
IMPORTS OF BENZENOID CHEMICALS, AND PRODUCTS 1977 United States General Imports of Intermediates, Dyes, Medicinals, Flavor and Perfume Materials, and Other Finished Benzenoid Products Entered in 1977 Under Schedule 4, Part 1, of the . Tariff Schedules of the United States USITC PUBLICATION 900 . JULY 1978 United States International Trade Commission I Washington, D.C. 20436 UNITED ST ATES INTERNATIONAL· TRADE. COMMISSION COMMISSIONERS Joseph 0. Parker, Chairman Bill Alberger, Vice Chairman George M. Moore Catherine Bedell ltalo H. Ablondi Daniel Minchew Kenneth R. Mason, Secretary to the Commission OFFICE OF INDUSTRIES Norris A. Lynch, Director This report was principally prepared by Frances Battle, Virginia Bailey, Judy Bryant, Ralph Gray, Sharon Greenfield, Mildred Higgs, Kenneth Kozel, Ross Lewis, Linda Mudd, and Loretta Willis of the Energy and Chemical Division. Antomatic Data Processing imput was provided by James Gill and Dean Stout. Address all communications to Office of the Secretary United States International Trade Commission Washington, D.C. 20436 For Release Contact: Mr. James O'Connor July 21, 1978 (202) 523-0496 USITC 78-076 UNITED STATES INTERNATIONAL TRADE COMMISSION RELEASES FIGURES ON IMPORTS OF BENZENOID CHEMICALS AND PRODUCTS IN 1977 The U.S. International Trade Commission today issued a re~ port on U.S. imports of benzenoid chemicals and products in 1977. The report includes data on articles entered under schedule 4, parts lB and lC, of the Tariff Schedules of the United States. It provides detailed statistics on imports of 2,670 products, including benzenoid intermediates, dyes, organic pigments, medic inals and pharmaceuticals, flavor and pe~fume materials, and other benzenoid products. -

Dibenzothiophene (DBT), and Carbazole (CA) from Benzofuran (BF), Benzothiophene (BT), and Indole (IN) with Cyclopentadienyl Radical
International Journal of Molecular Sciences Article The Gas-Phase Formation Mechanism of Dibenzofuran (DBF), Dibenzothiophene (DBT), and Carbazole (CA) from Benzofuran (BF), Benzothiophene (BT), and Indole (IN) with Cyclopentadienyl Radical 1, 1, 1,2 1 1,2, 3 Xuan Li y, Yixiang Gao y, Chenpeng Zuo , Siyuan Zheng , Fei Xu *, Yanhui Sun and Qingzhu Zhang 1 1 Environment Research Institute, Shandong University, Qingdao 266237, China; [email protected] (X.L.); [email protected] (Y.G.); [email protected] (C.Z.); [email protected] (S.Z.); [email protected] (Q.Z.) 2 Shenzhen Research Institute, Shandong University, Shenzhen 518057, China 3 College of Environment and Safety Engineering, Qingdao University of Science & Technology, Qingdao 266042, China; [email protected] * Correspondence: [email protected]; Tel.: +86-532-58631992 These authors contributed equally to this article. y Received: 11 August 2019; Accepted: 28 October 2019; Published: 31 October 2019 Abstract: Benzofuran (BF), benzothiophene (BT), indole (IN), dibenzofuran (DBF), dibenzothiophene (DBT), and carbazole (CA) are typical heterocyclic aromatic compounds (NSO-HETs), which can coexist with polycyclic aromatic hydrocarbons (PAHs) in combustion and pyrolysis conditions. In this work, quantum chemical calculations were carried out to investigate the formation of DBF, DBT, and CA from the reactions of BF, BT, and IN with a cyclopentadienyl radical (CPDyl) by using the hybrid density functional theory (DFT) at the MPWB1K/6-311+G(3df,2p)//MPWB1K/6-31+G(d,p) level. The rate constants of crucial elementary steps were deduced over 600 1200 K, using canonical − variational transition state theory with a small-curvature tunneling contribution (CVT/SCT).