Local, Regional and Monographic Approaches to Cyrtandra (Gesneriaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Henckelia Pradeepiana, a New Species of Gesneriaceae from the Southern Western Ghats, India
Rheedea Vol. 22(2) 119-123 2012 Henckelia pradeepiana, a new species of Gesneriaceae from the southern Western Ghats, India K.M. Manudev, A. Weber1 and Santhosh Nampy* Plant Systematics & Floristics Lab, Department of Botany, St. Joseph’s College, Devagiri, Kozhikode – 673 008, Kerala, India. 1Department of Structural and Functional Botany, Faculty of Biodiversity,University of Vienna, Rennweg 14, A-1030 Vienna, Austria. *E-mail: [email protected] Abstract A new species of Gesneriaceae, Henckelia pradeepiana, is described from the southern Western Ghats, India. The species is remarkable by the presence of flat tubers, from which shoots with a single or few basal leaves and large and lax inflorescences (pair-flowered cymes) emerge. The corolla is white or pale violet and obliquely campanulate. Also remarkable is the bright yellow stigma with strongly expanded and sometimes slightly emarginate lower lip. This “chiritoid” stigma form supports the recent inclusion of most species of Chirita sect. Chirita into the newly defined genusHenckelia . The closest relative of H. pradeepiana is probably H. missionis, known from the Western Ghats of Kanyakumari district, Tamil Nadu, some 400 km away from the former. Keywords: Gesneriaceae, Henckelia pradeepiana, New Species, Western Ghats Introduction During the preparation of a botanical inventory South Indian and Sri Lankan floras (Gamble, 1924; on Vellarimala, a floristically rich hill tract of Theobald & Grupe, 1981; Nayar et al., 2006). Western Ghats of Kerala, Dr. A.K. Pradeep first Henckelia pradeepiana Nampy, Manudev et A. collected unidentified single-leaved gesneriad Weber, sp. nov. Figs 1, 2 specimens in 1997. They were found in a small population comprising a few plants on a stream- Henckeliae missionis similis et probabiliter affinis, side damp rock near a waterfall at Olichuchattam sed (inter alia) habitu subunifoliato et fructibus at an elevation of 1160 m. -

Further Miscellaneous Species of Cyrtandra in Borneo
E D I N B U R G H J O U R N A L O F B O T A N Y 63 (2&3): 209–229 (2006) 209 doi:10.1017/S0960428606000564 E Trustees of the Royal Botanic Garden Edinburgh (2006) Issued 30 November 2006 OLD WORLD GESNERIACEAE XII: FURTHER MISCELLANEOUS SPECIES OF CYRTANDRA IN BORNEO O. M. HILLIARD &B.L.BURTT Nineteen miscellaneous species of Bornean Cyrtandra are dealt with. Cyrtandra atrichoides, C. congestiflora, C. crockerella, C. dulitiana, C. kanae, C. libauensis, C. plicata, C. vaginata and C. disparoides subsp. inconspicua are newly described. Descriptions and discussion are provided for C. erythrotricha and C. poulsenii, originally published with diagnoses only. Cyrtandra axillaris, C. longicarpa and C. microcarpa are also described, while C. borneensis, C. dajakorum, C. glomeruliflora, C. latens and C. prolata are reduced to synonymy. Keywords. Borneo, Cyrtandra, Gesneriaceae, new species. I NTRODUCTION A good many species of Cyrtandra in Borneo still remain undescribed; some available specimens are known to us only in the sterile state or are otherwise inadequate to typify a name. In this paper eight species and one subspecies are newly described. Burtt (1996) published new species with diagnoses only: C. erythrotricha B.L.Burtt and C. poulsenii B.L.Burtt are now fully described while C. glomeruliflora B.L.Burtt is reduced to synonymy under C. poulsenii. Full descriptions of C. axillaris C.B.Clarke and C. microcarpa C.B.Clarke are also given for the first time with the reduction of C. latens C.B.Clarke and C. dajakorum Kraenzl. -

Polygalaceae) from Borneo
Gardens' Bulletin Singapore 57 (2005) 47–61 47 New Taxa and Taxonomic Status in Xanthophyllum Roxb. (Polygalaceae) from Borneo W.J.J.O. DE WILDE AND BRIGITTA E.E. DUYFJES National Herbarium of the Netherlands, Leiden Branch P.O. Box 9514, 2300 RA Leiden, The Netherlands Abstract Thirteen new taxa or taxa with a new status in Xanthophyllum (Polygalaceae) from Borneo are described. The ten new species described in this paper are: X. bicolor W.J. de Wilde & Duyfjes, X. brachystachyum W.J. de Wilde & Duyfjes, X. crassum W.J. de Wilde & Duyfjes, X. inflatum W.J. de Wilde & Duyfjes, X. ionanthum W.J. de Wilde & Duyfjes, X. longum W.J. de Wilde & Duyfjes, X. nitidum W.J. de Wilde & Duyfjes, X. pachycarpon W.J. de Wilde & Duyfjes, X. rectum W.J. de Wilde & Duyfjes and X. rheophilum W.J. de Wilde & Duyfjes, and the new variety is X. griffithii A.W. Benn var. papillosum W.J. de Wilde & Duyfjes. New taxonomic status has been accorded to X. adenotus Miq. var. arsatii (C.E.C. Fisch.) W.J. de Wilde & Duyfjes and X. lineare (Meijden) W.J. de Wilde & Duyfjes. Introduction During the study of Xanthophyllum carried out in the BO, KEP, L, SAN, SAR and SING herbaria for the account of Polygalaceae in the Tree Flora of Sabah and Sarawak, several new taxa were defined. Their taxonomic position within the more than 50 species of Xanthophyllum recognised in Sabah and Sarawak will be clarified in the treatment of the family in the forthcoming volume of the Tree Flora of Sabah and Sarawak series. -

Ekspedisi Saintifik Biodiversiti Hutan Paya Gambut Selangor Utara 28 November 2013 Hotel Quality, Shah Alam SELANGOR D
Prosiding Ekspedisi Saintifik Biodiversiti Hutan Paya Gambut Selangor Utara 28 November 2013 Hotel Quality, Shah Alam SELANGOR D. E. Seminar Ekspedisi Saintifik Biodiversiti Hutan Paya Gambut Selangor Utara 2013 Dianjurkan oleh Jabatan Perhutanan Semenanjung Malaysia Jabatan Perhutanan Negeri Selangor Malaysian Nature Society Ditaja oleh ASEAN Peatland Forest Programme (APFP) Dengan Kerjasama Kementerian Sumber Asli and Alam Sekitar (NRE) Jabatan Perlindungan Hidupan Liar dan Taman Negara (PERHILITAN) Semenanjung Malaysia PROSIDING 1 SEMINAR EKSPEDISI SAINTIFIK BIODIVERSITI HUTAN PAYA GAMBUT SELANGOR UTARA 2013 ISI KANDUNGAN PENGENALAN North Selangor Peat Swamp Forest .................................................................................................. 2 North Selangor Peat Swamp Forest Scientific Biodiversity Expedition 2013...................................... 3 ATURCARA SEMINAR ........................................................................................................................... 5 KERTAS PERBENTANGAN The Socio-Economic Survey on Importance of Peat Swamp Forest Ecosystem to Local Communities Adjacent to Raja Musa Forest Reserve ........................................................................................ 9 Assessment of North Selangor Peat Swamp Forest for Forest Tourism ........................................... 34 Developing a Preliminary Checklist of Birds at NSPSF ..................................................................... 41 The Southern Pied Hornbill of Sungai Panjang, Sabak -

Aeschynanthus Pulcher Lipstick Plant LIPSTICK PLANT
The Gardener’s Resource 435 W. Glenside Ave. Since 1943 Glenside, PA 19038 215-887-7500 Aeschynanthus pulcher Lipstick Plant LIPSTICK PLANT Lipstick plants are easy indoor flowering houseplants that when given the right amount of light and water, they produce numerous red or orange small tubular flowers that resemble a tube of lipstick. Not only are the flowers colorful, the leaves can be light green, dark green or green and maroon. Light: The Lipstick vine will not bloom without adequate light. They require very bright, indirect light. Avoid placing this plant in full shade or full sun. Direct sun will burn the leaves. The plant needs bright light for a portion of the day, but not all day long. Water: If you allow the top 25% of the soil to dry out before watering, this plant will flower more frequently and more abundant. If the leaves appear soft and shriveled, give it more water. These plants will also lose green leaves TIPS: when over-watered. • Lipstick plants like warm temperatures between 75-85°F. : Fertilizer every other week in the Fertilizing • Prefers high humidity, but will do well in Spring and Summer and only monthly in the basic household humidity too. Fall and Winter with a houseplant food high in • Trim the long vines to prevent the plant phosphorous. Always dilute the fertilizer to ½ from becoming thin and straggly. the recommended strength. • Lipstick Plants are Non-Poisonous. • Keep a lipstick plant in a small pot will help it produce more flowers. When put into a large container, instead of producing flowers it grows more leaves. -

TITLE Fulbright-Hays Seminars Abroad Program: Malaysia 1995
DOCUMENT RESUME ED 405 265 SO 026 916 TITLE Fulbright-Hays Seminars Abroad Program: Malaysia 1995. Participants' Reports. INSTITUTION Center for International Education (ED), Washington, DC.; Malaysian-American Commission on Educational Exchange, Kuala Lumpur. PUB DATE 95 NOTE 321p.; Some images will not reproduce clearly. PUB TYPE Guides Non-Classroom Use (055) Reports Descriptive (141) Collected Works General (020) EDRS PRICE MFO1 /PC13 Plus Postage. DESCRIPTORS Area Studies; *Asian History; *Asian Studies; Cultural Background; Culture; Elementary Secondary Education; Foreign Countries; Foreign Culture; *Global Education; Human Geography; Instructional Materials; *Non Western Civilization; Social Studies; *World Geography; *World History IDENTIFIERS Fulbright Hays Seminars Abroad Program; *Malaysia ABSTRACT These reports and lesson plans were developed by teachers and coordinators who traveled to Malaysia during the summer of 1995 as part of the U.S. Department of Education's Fulbright-Hays Seminars Abroad Program. Sections of the report include:(1) "Gender and Economics: Malaysia" (Mary C. Furlong);(2) "Malaysia: An Integrated, Interdisciplinary Social Studies Unit for Middle School/High School Students" (Nancy K. Hof);(3) "Malaysian Adventure: The Cultural Diversity of Malaysia" (Genevieve M. Homiller);(4) "Celebrating Cultural Diversity: The Traditional Malay Marriage Ritual" (Dorene H. James);(5) "An Introduction of Malaysia: A Mini-unit for Sixth Graders" (John F. Kennedy); (6) "Malaysia: An Interdisciplinary Unit in English Literature and Social Studies" (Carol M. Krause);(7) "Malaysia and the Challenge of Development by the Year 2020" (Neale McGoldrick);(8) "The Iban: From Sea Pirates to Dwellers of the Rain Forest" (Margaret E. Oriol);(9) "Vision 2020" (Louis R. Price);(10) "Sarawak for Sale: A Simulation of Environmental Decision Making in Malaysia" (Kathleen L. -

<I>Loxocarpus Pauzii</I> (<I>Gesneriaceae</I>), a New
Blumea 57, 2012: 134–135 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE http://dx.doi.org/10.3767/000651912X657512 Loxocarpus pauzii (Gesneriaceae), a new species from Peninsular Malaysia T.L. Yao1, 2, R. Kiew1, N.W. Haron2 Key words Abstract A new species of Loxocarpus (Gesneriaceae) from Gunung Stong, Kelantan, Peninsular Malaysia, is described and illustrated. Gesneriaceae Loxocarpus Published on 14 September 2012 new species Peninsular Malaysia INTRODUCTION Etymology. This species is named after Pauzi Husin (1976–2011), a nature guide who first discovered it. A Loxocarpus R.Br. (Gesneriaceae) was discovered during Rosette plant. Rootstock short, woody, to 3 cm long, 5 mm a botanical expedition to the Gunung Tera area, Kelantan, diam, with wiry adventitious roots. Indumentum of stem and Peninsular Malaysia, in February 2007 (Chew et al. 2007). leaves silvery when dry, mainly of straight uniseriate, multicel- The plant was growing on the wet, dripping surface of a large lular silky non-glandular hairs: on rootstock dense, 1–1.5 mm granite boulder beside the Stong Waterfall. Although sterile, its long, on the petiole very dense, 1–2 mm long, on the upper leaves were so different from any known Loxocarpus species surface of the lamina dense, c. 0.85 mm long, on the veins of that living plants were collected and grown in the Kepong Bo- the lower surface of the lamina less dense, 0.4–0.85 mm long, tanic Garden nursery, Forest Research Institute Malaysia. On on the lower surface of the lamina sparse, c. 0.3 mm long, on flowering, unique characters of its flower confirmed its status the bracts and bracteoles dense, 0.3 mm long; indumentum of as a new species. -

The Provider-Based Evaluation (Probe) 2014 Preliminary Report
The Provider-Based Evaluation (ProBE) 2014 Preliminary Report I. Background of ProBE 2014 The Provider-Based Evaluation (ProBE), continuation of the formerly known Malaysia Government Portals and Websites Assessment (MGPWA), has been concluded for the assessment year of 2014. As mandated by the Government of Malaysia via the Flagship Coordination Committee (FCC) Meeting chaired by the Secretary General of Malaysia, MDeC hereby announces the result of ProBE 2014. Effective Date and Implementation The assessment year for ProBE 2014 has commenced on the 1 st of July 2014 following the announcement of the criteria and its methodology to all agencies. A total of 1086 Government websites from twenty four Ministries and thirteen states were identified for assessment. Methodology In line with the continuous and heightened effort from the Government to enhance delivery of services to the citizens, significant advancements were introduced to the criteria and methodology of assessment for ProBE 2014 exercise. The year 2014 spearheaded the introduction and implementation of self-assessment methodology where all agencies were required to assess their own websites based on the prescribed ProBE criteria. The key features of the methodology are as follows: ● Agencies are required to conduct assessment of their respective websites throughout the year; ● Parents agencies played a vital role in monitoring as well as approving their agencies to be able to conduct the self-assessment; ● During the self-assessment process, each agency is required to record -

Of ODOARDOBECCARI Dedication
1864 1906 1918 Dedicated to the memory of ODOARDOBECCARI Dedication A dedication to ODOARDO BECCARI, the greatest botanist ever to study in Malesia, is long overdue. Although best known as a plant taxonomist, his versatile genius extended far beyond the basic field ofthis branch ofBotany, his wide interest leading him to investigate the laws ofevolution, the interrelations between plants and animals, the connection between vegetation and environ- cultivated ment, plant distribution, the and useful plants of Malesia and many other problems of life. plant But, even if he devoted his studies to plants, in the depth of his mind he was primarily a naturalist, and in his long, lonely and dangerousexplorations in Malesia he was attracted to all aspects ofnature and human life, assembling, besides plants, an incredibly large number of collec- tions and an invaluable wealth ofdrawings and observations in zoology, anthropologyand ethnol- He ogy. was indeed a naturalist, and one of the greatest of his time; but never in his mind were the knowledge and beauty of Nature disjoined, and, as he was a true and complete naturalist, he the time was at same a poet and an artist. His Nelleforestedi Borneo, Viaggi ericerche di un mturalista(1902), excellently translatedinto English (in a somewhatabbreviated form) by Prof. E. GioLiouandrevised and edited by F.H.H. Guillemardas Wanderingsin the great forests of Borneo (1904), is a treasure in tropical botany; it is in fact an unrivalledintroductionto tropical plant lifeand animals, man included. It is a most readable book touching on all sorts of topics and we advise it to be studied by all young people whose ambition it is to devote their life to tropical research. -

GROWING INDOOR PLANTS with Success
GROWING INDOOR PLANTS with Success Table of Contents Introduction............................................................................................................................ 3 Factors Affecting Plant Growth............................................................................................ 3 Light..................................................................................................................................... 3 Temperature........................................................................................................................ 5 Relative Humidity................................................................................................................. 6 Water................................................................................................................................... 7 Water Quantity ................................................................................................................. 7 Water Quality.................................................................................................................... 7 Nutrition ............................................................................................................................... 8 Soil/Growing Medium .......................................................................................................... 9 Growing Mix for Flowering House Plants.......................................................................... 9 Growing Mixes for Foliage Plants.................................................................................... -

An Early History of Vireya the People, Places & Plants of the Nineteenth Century
An Early History of Vireya The People, Places & Plants of the Nineteenth Century Chris Callard The distribution of Rhododendron subgenus Vireya is centred on the botanical region known as Malesia – an area of south-east Asia encompassing the Malay Archipelago, the Philippines, Borneo, Indonesia and New Guinea and surrounding island groups. It is for this reason that vireyas have sometimes in the past been referred to as ‘Malesian Rhododendrons’, although nowadays this is not considered a strictly accurate term as a small number of the 318 species in subgenus Vireya grow outside this region and, similarly, a few species from other subgenera of Rhododendron are also to be found within its boundaries. Broadly speaking, the Vireya group extends from Taiwan in the north to Queensland, Australia in the south, and from India in the west to the Solomon Islands in the east. Much of the early recorded history of the plants of Rhododendron subgenus Vireya came about as a result of the activities of European nations, particularly Britain and Holland, pursuing their colonial ambitions across the Malay Archipelago and, later, east to New Guinea. As their Empires expanded into these previously unexplored territories, settlements were established and expeditions mounted to survey the natural wealth and geography of the land. Many of these early explorers had scientific backgrounds, although not always in botany, and collected all manner of exotic flora and fauna found in these unfamiliar surroundings, to ship back to their homelands. The Vireya story starts in June 1821 when the Scotsman, William Jack, one of “a party of gentlemen”i, set out from Bencoolen (now Bengkulu), a settlement on the south-west coast of Sumatra, to reach the summit of Gunong Benko (Bungkuk), the so-called Sugar Loaf Mountain, “not estimated to exceed 3,000 feet in height”. -
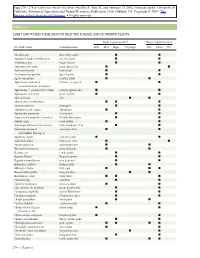
Light and Water Guidelines for Selected Foliage and Flowering Plants
Table 11.1 LIGHT AND WATER GUIDELINES FOR SELECTED FOLIAGE AND FLOWERING PLANTS Light requirements* Water requirements† Scientific name Common name Low Med High Very high Dry Moist Wet Abutilon spp. flowering maple II Acalypha hispida (A.wilkesiana) chenille plant I I Achimenes spp. magic flower II Adiantum cuneatum maidenhair fern II Aechmea fasciata bromeliad II Aeschynanthus pulcher lipstick plant II Agave americana century plant II Aglaonema modestum Chinese evergreen II (A.commutatum,A.simplex) Aglaonema ϫ pseudo-bracteatum golden aglaonema II Aglaonema roebelenii pewter plant II Aloe variegata aloe II Alternanthera bettzickiana II I Ananas comosus pineapple I I Anthurium andreanum anthurium II Aphelandra squarrosa zebra plant II Araucaria heterophylla (A.excelsa) Norfolk Island pine II Ardisia crispa coral ardisia II Asparagus plumosus (A.setaceus) bride’s bouquet fern II Asparagus sprengeri asparagus fern II (A.densiflora Sprenger) Aspidistra elatior cast-iron plant I I Asplenium nidus bird’s nest fern I I Aucuba japonica gold-dust plant I I Beaucarnea recurvata pony tail palm I I Begonia rex rex begonia I I Begonia ‘Rieger’ Rieger begonia I I Begonia semperflorens wax begonia II Beloperone guttata shrimp plant II Billbergia zebrina billbergia III Bougainvillea glabra bougainvillea II Browallia speciosa bush violet II I Caladium spp. caladium II Calathea makoyana peacock plant II Calceolaria herbeahybrida pocketbook plant II Campanula isophylla star-of-Bethlehem II Capsicum annuum Christmas pepper II Carissa grandiflora Natal plum