October 20, 2015 Ms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lifetime Organophosphorous Insecticide Use Among Private Pesticide Applicators in the Agricultural Health Study
Journal of Exposure Science and Environmental Epidemiology (2012) 22, 584 -- 592 & 2012 Nature America, Inc. All rights reserved 1559-0631/12 www.nature.com/jes ORIGINAL ARTICLE Lifetime organophosphorous insecticide use among private pesticide applicators in the Agricultural Health Study Jane A. Hoppin1, Stuart Long2, David M. Umbach3, Jay H. Lubin4, Sarah E. Starks5, Fred Gerr5, Kent Thomas6, Cynthia J. Hines7, Scott Weichenthal8, Freya Kamel1, Stella Koutros9, Michael Alavanja9, Laura E. Beane Freeman9 and Dale P. Sandler1 Organophosphorous insecticides (OPs) are the most commonly used insecticides in US agriculture, but little information is available regarding specific OP use by individual farmers. We describe OP use for licensed private pesticide applicators from Iowa and North Carolina in the Agricultural Health Study (AHS) using lifetime pesticide use data from 701 randomly selected male participants collected at three time periods. Of 27 OPs studied, 20 were used by 41%. Overall, 95% had ever applied at least one OP. The median number of different OPs used was 4 (maximum ¼ 13). Malathion was the most commonly used OP (74%) followed by chlorpyrifos (54%). OP use declined over time. At the first interview (1993--1997), 68% of participants had applied OPs in the past year; by the last interview (2005--2007), only 42% had. Similarly, median annual application days of OPs declined from 13.5 to 6 days. Although OP use was common, the specific OPs used varied by state, time period, and individual. Much of the variability in OP use was associated with the choice of OP, rather than the frequency or duration of application. -

Table II. EPCRA Section 313 Chemical List for Reporting Year 2017 (Including Toxic Chemical Categories)
Table II. EPCRA Section 313 Chemical List For Reporting Year 2017 (including Toxic Chemical Categories) Individually listed EPCRA Section 313 chemicals with CAS numbers are arranged alphabetically starting on page II-3. Following the alphabetical list, the EPCRA Section 313 chemicals are arranged in CAS number order. Covered chemical categories follow. Note: Chemicals may be added to or deleted from the list. The Emergency Planning and Community Right-to-Know Call Center or the TRI-Listed Chemicals website will provide up-to-date information on the status of these changes. See section B.3.c of the instructions for more information on the de minimis % limits listed below. There are no de minimis levels for PBT chemicals since the de minimis exemption is not available for these chemicals (an asterisk appears where a de minimis limit would otherwise appear in Table II). However, for purposes of the supplier notification requirement only, such limits are provided in Appendix C. Chemical Qualifiers Certain EPCRA Section 313 chemicals listed in Table II have parenthetic “qualifiers.” These qualifiers indicate that these EPCRA Section 313 chemicals are subject to the section 313 reporting requirements if manufactured, processed, or otherwise used in a specific form or when a certain activity is performed. An EPCRA Section 313 chemical that is listed without a qualifier is subject to reporting in all forms in which it is manufactured, processed, and otherwise used. The following chemicals are reportable only if they are manufactured, processed, or otherwise used in the specific form(s) listed below: Chemical/ Chemical Category CAS Number Qualifier Aluminum (fume or dust) 7429-90-5 Only if it is a fume or dust form. -

Organophosphate Insecticides
CHAPTER 4 HIGHLIGHTS Organophosphate Insecticides Acts through phosphorylation of the acetylcholinesterase enzyme Since the removal of organochlorine insecticides from use, organophosphate at nerve endings insecticides have become the most widely used insecticides available today. More Absorbed by inhalation, than forty of them are currently registered for use and all run the risk of acute ingestion, and skin and subacute toxicity. Organophosphates are used in agriculture, in the home, penetration in gardens, and in veterinary practice. All apparently share a common mecha- Muscarinic, nicotinic & CNS nism of cholinesterase inhibition and can cause similar symptoms. Because they effects share this mechanism, exposure to the same organophosphate by multiple routes or to multiple organophosphates by multiple routes can lead to serious additive Signs and Symptoms: toxicity. It is important to understand, however, that there is a wide range of Headache, hypersecretion, toxicity in these agents and wide variation in cutaneous absorption, making muscle twitching, nausea, specific identification and management quite important. diarrhea Respiratory depression, seizures, loss of consciousness Toxicology Miosis is often a helpful Organophosphates poison insects and mammals primarily by phosphory- diagnostic sign lation of the acetylcholinesterase enzyme (AChE) at nerve endings. The result is a loss of available AChE so that the effector organ becomes overstimulated by Treatment: the excess acetylcholine (ACh, the impulse-transmitting substance) in the nerve Clear airway, improve tissue ending. The enzyme is critical to normal control of nerve impulse transmission oxygenation from nerve fibers to smooth and skeletal muscle cells, glandular cells, and Administer atropine sulfate autonomic ganglia, as well as within the central nervous system (CNS). -

Innovative Biocatalysts As Tools to Detect and Inactivate Nerve Agents Elena Porzio1, Francesca Bettazzi2, Luigi Mandrich1, Immacolata Del Giudice1, Odile F
www.nature.com/scientificreports OPEN Innovative Biocatalysts as Tools to Detect and Inactivate Nerve Agents Elena Porzio1, Francesca Bettazzi2, Luigi Mandrich1, Immacolata Del Giudice1, Odile F. Restaino3, Serena Laschi4, Ferdinando Febbraio1, Valentina De Luca1, 3 1 5 6 Received: 24 February 2018 Maria G. Borzacchiello , Teresa M. Carusone , Franz Worek , Antonio Pisanti , Piero Porcaro6, Chiara Schiraldi3, Mario De Rosa3, Ilaria Palchetti 2 & Giuseppe Manco1 Accepted: 25 July 2018 Published: xx xx xxxx Pesticides and warfare nerve agents are frequently organophosphates (OPs) or related compounds. Their acute toxicity highlighted more than ever the need to explore applicable strategies for the sensing, decontamination and/or detoxifcation of these compounds. Herein, we report the use of two diferent thermostable enzyme families capable to detect and inactivate OPs. In particular, mutants of carboxylesterase-2 from Alicyclobacillus acidocaldarius and of phosphotriesterase-like lactonases from Sulfolobus solfataricus and Sulfolobus acidocaldarius, have been selected and assembled in an optimized format for the development of an electrochemical biosensor and a decontamination formulation, respectively. The features of the developed tools have been tested in an ad-hoc fabricated chamber, to mimic an alarming situation of exposure to a nerve agent. Choosing ethyl-paraoxon as nerve agent simulant, a limit of detection (LOD) of 0.4 nM, after 5 s of exposure time was obtained. Furthermore, an optimized enzymatic formulation was used for a fast and efcient environmental detoxifcation (>99%) of the nebulized nerve agent simulants in the air and on surfaces. Crucial, large- scale experiments have been possible thanks to production of grams amounts of pure (>90%) enzymes. Pesticides and warfare nerve agents are frequently organophosphates (OPs) or related compounds (e.g. -

Current Awareness in Clinical Toxicology Editors: Damian Ballam Msc and Allister Vale MD
Current Awareness in Clinical Toxicology Editors: Damian Ballam MSc and Allister Vale MD January 2017 CONTENTS General Toxicology 11 Metals 38 Management 21 Pesticides 39 Drugs 23 Chemical Warfare 41 Chemical Incidents & 33 Plants 41 Pollution Chemicals 33 Animals 42 CURRENT AWARENESS PAPERS OF THE MONTH 2015 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 33rd Annual Report Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. Clin Toxicol 2016; 54: 924-1109. Introduction This is the 33rd Annual Report of the American Association of Poison Control Centers' (AAPCC) National Poison Data System (NPDS). As of 1 January 2015, 55 of the nation's poison centers (PCs) uploaded case data automatically to NPDS. The upload interval was 9.52 [7.40, 13.6] (median [25%, 75%]) minutes, creating a near real-time national exposure and information database and surveillance system. Methods We analyzed the case data tabulating specific indices from NPDS. The methodology was similar to that of previous years. Where changes were introduced, the differences are identified. Poison center cases with medical outcomes of death were evaluated by a team of medical and clinical toxicologist reviewers using an ordinal scale of 1-6 to assess the Relative Contribution to Fatality (RCF) of the exposure. Results In 2015, 2,792,130 closed encounters were logged by NPDS: 2,168,371 human exposures, 55,516 animal exposures, 560,467 information calls, 7657 human confirmed nonexposures, Current Awareness in Clinical Toxicology is produced monthly for the American Academy of Clinical Toxicology by the Birmingham Unit of the UK National Poisons Information Service, with contributions from the Cardiff, Edinburgh, and Newcastle Units. -

Environmental Health Criteria 63 ORGANOPHOSPHORUS
Environmental Health Criteria 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION Please note that the layout and pagination of this web version are not identical with the printed version. Organophophorus insecticides: a general introduction (EHC 63, 1986) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1986 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination -

Recent Advances on Detection of Insecticides Using Optical Sensors
sensors Review Recent Advances on Detection of Insecticides Using Optical Sensors Nurul Illya Muhamad Fauzi 1, Yap Wing Fen 1,2,*, Nur Alia Sheh Omar 1,2 and Hazwani Suhaila Hashim 2 1 Functional Devices Laboratory, Institute of Advanced Technology, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; [email protected] (N.I.M.F.); [email protected] (N.A.S.O.) 2 Department of Physics, Faculty of Science, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; [email protected] * Correspondence: [email protected] Abstract: Insecticides are enormously important to industry requirements and market demands in agriculture. Despite their usefulness, these insecticides can pose a dangerous risk to the safety of food, environment and all living things through various mechanisms of action. Concern about the environmental impact of repeated use of insecticides has prompted many researchers to develop rapid, economical, uncomplicated and user-friendly analytical method for the detection of insecticides. In this regards, optical sensors are considered as favorable methods for insecticides analysis because of their special features including rapid detection time, low cost, easy to use and high selectivity and sensitivity. In this review, current progresses of incorporation between recognition elements and optical sensors for insecticide detection are discussed and evaluated well, by categorizing it based on insecticide chemical classes, including the range of detection and limit of detection. Additionally, this review aims to provide powerful insights to researchers for the future development of optical sensors in the detection of insecticides. Citation: Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Hashim, H.S. Recent Keywords: insecticides; optical sensor; recognition element Advances on Detection of Insecticides Using Optical Sensors. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

Tetrachlorvinphos
Common Name: TETRACHLORVINPHOS CAS Number: 961-11-5 RTK Substance number: 1813 DOT Number: UN 2783 Date: September 2000 ----------------------------------------------------------------------- ----------------------------------------------------------------------- HAZARD SUMMARY * Tetrachlorvinphos can affect you when breathed in and * If you think you are experiencing any work-related health quickly enters the body by passing through the skin. problems, see a doctor trained to recognize occupational * Contact can irritate the skin and eyes. diseases. Take this Fact Sheet with you. * Exposure to Tetrachlorvinphos can cause rapid, FATAL Organophosphate poisoning with headache, sweating, WORKPLACE EXPOSURE LIMITS nausea and vomiting, diarrhea, loss of coordination, and No occupational exposure limits have been established for death. Tetrachlorvinphos. This does not mean that this substance is * Breathing Tetrachlorvinphos can irritate the lungs not harmful. Safe work practices should always be followed. causing coughing and/or shortness of breath. Higher exposures can cause a build-up of fluid in the lungs * It should be recognized that Tetrachlorvinphos can be (pulmonary edema), a medical emergency, with severe absorbed through your skin, thereby increasing your shortness of breath. exposure. * Tetrachlorvinphos may cause a skin allergy. If allergy develops, very low future exposure can cause itching and a WAYS OF REDUCING EXPOSURE skin rash. * Where possible, enclose operations and use local exhaust * Repeated exposure may cause personality changes of ventilation at the site of chemical release. If local exhaust depression, anxiety or irritability. ventilation or enclosure is not used, respirators should be worn. IDENTIFICATION * Wear protective work clothing. Tetrachlorvinphos is a colorless or white powder. It is used * Wash thoroughly immediately after exposure to as an insecticide. Tetrachlorvinphos and at the end of the workshift. -
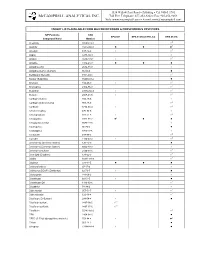
Compounds/Target Lists Detail
1534 Willow Pass Road • Pittsburg • CA 94565-1701 McCAMPBELL ANALYTICAL INC. Toll Free Telephone: 877-252-9262 • Fax: 925-252-9269 Web: www.mccampbell.com • E-mail: [email protected] TARGET LISTS AVAILABLE FROM MAI FOR NITROGEN & PHOSPHOROUS PESTICIDES N/P Pesticide CAS EPA 507 EPA 8141A (CTR List) EPA 8141A Compound Name Number Acephate 30560-19-1 zZ Alachlor 15972-60-8 z z zZ Ametryn 834-12-8 z zZ Aspon 3244-90-4 z Atraton 1610-17-9 z zZ Atrazine 1912-24-9 z z z Azinphos ethyl 2642-71-9 z Azinphos methyl (Guthion) 86-50-0 z Benfluralin (Benefin) 1861-40-1 zZ Bolstar (Sulprofos) 35400-43-2 z Bromacil 314-40-9 z zZ Bromophos 2104-96-3 zZ Butachlor 23184-66-9 z zZ Butylate 2008-41-5 z zZ Carbophenothion 786-19-6 z Carbophenothion methyl 953-17-3 zZ Carboxin 5234-68-4 z zZ Chlorfenvinphos 470-90-6 z Chloropropham 101-21-3 z zZ Chlorpyrifos 2921-88-2 zZ z z Chlorpyrifos methyl 5598-13-0 z Coumaphos 56-72-4 z Crotoxyphos 7700-17-6 z Crufomate 299-86-5 zZ Cycloate 1134-23-2 z zZ Demeton-O (Demeton isomer) 126-75-0 z Demeton-S (Demeton isomer) 8065-48-3 z Demeton-S sulfone 2496-91-5 zZ Deet (Off) (Dephere) 134-62-3 zZ Dialifor 10311-84-9 zZ Diazinon 333-41-5 z z z Dichlorofenthion 97-17-6 z Dichlorvos (DDVP) (Dichlorfos) 62-73-7 z z Dicrotophos 141-66-2 z Dimethoate 60-51-5 z z Dimethoate OA 1113-02-6 zZ Dioxathion 78-34-2 z Diphenamid 957-51-7 z zZ Diphenylamine 122-39-4 zZ Disulfoton (Di-Syston) 298-04-4 z z Disulfoton sulfone 2497-06-5 zX1 Disulfoton sulfoxide 2497-07-6 zX1 Ethalflurin 55283-68-6 zZ EPN 2104-64-5 z EPTC (S-Ethyl dipropylthiocarbamate) 759-94-4 z zZ Ethion 563-12-2 z Ethoprop 13194-48-4 z z 1534 Willow Pass Road • Pittsburg • CA 94565-1701 McCAMPBELL ANALYTICAL INC. -

Method 8270D: Semivolatile Organic Compounds by Gas
METHOD 8270D SEMIVOLATILE ORGANIC COMPOUNDS BY GAS CHROMATOGRAPHY/MASS SPECTROMETRY (GC/MS) SW-846 is not intended to be an analytical training manual. Therefore, method procedures are written based on the assumption that they will be performed by analysts who are formally trained in at least the basic principles of chemical analysis and in the use of the subject technology. In addition, SW-846 methods, with the exception of required method use for the analysis of method-defined parameters, are intended to be guidance methods which contain general information on how to perform an analytical procedure or technique which a laboratory can use as a basic starting point for generating its own detailed Standard Operating Procedure (SOP), either for its own general use or for a specific project application. The performance data included in this method are for guidance purposes only, and are not intended to be and must not be used as absolute QC acceptance criteria for purposes of laboratory accreditation. 1.0 SCOPE AND APPLICATION 1.1 This method is used to determine the concentration of semivolatile organic compounds in extracts prepared from many types of solid waste matrices, soils, air sampling media and water samples. Direct injection of a sample may be used in limited applications. The following RCRA analytes have been determined by this method: Appropriate Preparation Techniquesb 3540/ Compounds CAS Noa 3510 3520 3541 3550 3580 Acenaphthene 83-32-9 X X X X X Acenaphthylene 208-96-8 X X X X X Acetophenone 98-86-2 X ND ND ND X 2-Acetylaminofluorene -

IARC Monographs Volume 112: Evaluation of Five Organophosphate Insecticides and Herbicides
20 March 2015 IARC Monographs Volume 112: evaluation of five organophosphate insecticides and herbicides Lyon, France, 20 March 2015 – The International Agency for Research on Cancer (IARC), the specialized cancer agency of the World Health Organization, has assessed the carcinogenicity of five organophosphate pesticides. A summary of the final evaluations together with a short rationale have now been published online in The Lancet Oncology, and the detailed assessments will be published as Volume 112 of the IARC Monographs. What were the results of the IARC evaluations? The herbicide glyphosate and the insecticides malathion and diazinon were classified as probably carcinogenic to humans (Group 2A). The insecticides tetrachlorvinphos and parathion were classified as possibly carcinogenic to humans (Group 2B). What was the scientific basis of the IARC evaluations? The pesticides tetrachlorvinphos and parathion were classified as possibly carcinogenic to humans (Group 2B) based on convincing evidence that these agents cause cancer in laboratory animals. For the insecticide malathion, there is limited evidence of carcinogenicity in humans for non-Hodgkin lymphoma and prostate cancer. The evidence in humans is from studies of exposures, mostly agricultural, in the USA, Canada, and Sweden published since 2001. Malathion also caused tumours in rodent studies. Malathion caused DNA and chromosomal damage and also disrupted hormone pathways. For the insecticide diazinon, there was limited evidence of carcinogenicity in humans for non-Hodgkin lymphoma and lung cancer. The evidence in humans is from studies of agricultural exposures in the USA and Canada published since 2001. The classification of diazinon in Group 2A was also based on strong evidence that diazinon induced DNA or chromosomal damage.