Automated Billing Code Retrieval from MRI Scanner Log Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Omega-3 Fatty Acids As First-Line Treatment in Paediatric Depression
Se Clinical Study Protocol OMEGA-3 FATTY ACIDS AS FIRST-LINE TREATMENT IN PAEDIATRIC DEPRESSION. A phase III, 36-week, multi-centre, double-blind, placebo-controlled randomized superiority Study. The Omega-3-pMDD Study Study Type: Intervention with Investigational Medicinal Product (IMP) Study Categorisation: Clinical Trial with IMP Category C Study Registration: Swiss Federal Complementary Database Clinicaltrials.gov Study Identifier: SNF 33IC30_166826 Sponsor, Sponsor- Gregor Berger Investigator and Principal Investigator: Department of Child and Adolescent Psychiatry University Hospital of Psychiatry University of Zurich Neumünsterallee 9 Omega-3-pMDD, Version 3 of 13.07.2017 Page 1 of 108 CH 8032 Zürich Switzerland Phone: +41 43 499 26 71 Mobile: +41 76 464 61 54 E-Mail: [email protected] Investigational Product: Omega-3 fatty acids (1000mg EPA / 500mg DHA in > in 13 years old and 500mg EPA / 250mg DHA in < in 13 years old) Protocol Version and Version3 of 13..07.2017 Date: CONFIDENTIAL The information contained in this document is confidential and the property of the Department of Child and Adolescent Psychiatry of the University of Zurich. The information may not - in full or in part - be transmitted, reproduced, published, or disclosed to others than the applicable Independent Ethics Committee(s) and Competent Authority(ies) without prior written authorization from the Department of Child and Adolescent Psychiatry of the University of Zurich, except to the extent necessary to obtain informed consent from those participants who will participate in the study. Omega-3-pMDD, Version 3 of 13.07.2017 Page 2 of 108 SIGNATURE PAGES Study number Swiss Federal Complementary Database Study Title Omega-3 fatty acids as first-line treatment in Paediatric Depression. -

MHSUDS Information Notice No.: 16-051
State of California—Health and Human Services Agency Department of Health Care Services JENNIFER KENT EDMUND G. BROWN JR. DIRECTOR GOVERNOR DATE: October 7, 2016 MHSUDS INFORMATION NOTICE N O.: 16-051 TO: COUNTY BEHAVIORAL HEALTH DIRECTORS COUNTY BEHAVIORAL HEALTH DIRECTORS ASSOCIATION OF CALIFORNIA CALIFORNIA COUNCIL OF COMMUNITY BEHAVIORAL HEALTH AGENCIES CALIFORNIA ALLIANCE OF CHILD AND FAMILY SERVICES SUBJECT: IMPLEMENTATION OF THE DIAGNOSTIC AND STATISTICAL MANUAL OF MENTAL DISORDERS (DSM-5), FIFTH EDITION REFERENCES: MHSD INFORMATION NOTICE 13-22 MHSUDS INFORMATION NOTICE 14-040 MHSUDS INFORMATION NOTICE 15-003 MHSUDS INFORMATION NOTICE 15-030 PURPOSE The purpose of this Information Notice is to direct Mental Health Plans (MHPs) to use the American Psychiatric Association (APA) DS M-5 to make diagnostic determinations for the purposes of determining if beneficiaries meet medical necessity criteria for Medi-Cal specialty mental health services (SMHS). This Information Notice updates information previously provided in Mental Health Services Division (MHSD) Information Notice 13-22. BACKGROUND The APA issued the DSM-5 on May 20, 2013. The DSM-5 provides diagnostic criteria and codes as well as corresponding International Classification of Diseases-10 (ICD-10) codes. Since March 2013, the Department of Health Care Services (DHCS) has collaborated with internal and external stakeholders and partners to address implementation issues specific to SMHS related to the DSM-5. Mental Health & Substance Use Disorder Services 1501 Capitol Avenue, MS 4000, P.O. Box 997413 Sacramento, CA 95899-7413 Phone: (916) 440-7800 Fax: (916) 319-8219 Internet Address: www.dhcs.ca.gov MHSUDS INFORMATION NOTICE N O.: 16-051 October 7, 2016 Page 2 DHCS has issued previous MHSUDS Information Notices1 that instruct MHPs to report only ICD-10 codes for claiming and diagnoses reporting purposes and provide ICD-10 procedural and diagnosis crosswalk documents f or SMHS. -

ICD-10 International Statistical Classification of Diseases and Related Health Problems
ICD-10 International Statistical Classification of Diseases and Related Health Problems 10th Revision Volume 2 Instruction manual 2010 Edition WHO Library Cataloguing-in-Publication Data International statistical classification of diseases and related health problems. - 10th revision, edition 2010. 3 v. Contents: v. 1. Tabular list – v. 2. Instruction manual – v. 3. Alphabetical index. 1.Diseases - classification. 2.Classification. 3.Manuals. I.World Health Organization. II.ICD-10. ISBN 978 92 4 154834 2 (NLM classification: WB 15) © World Health Organization 2011 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/copyright_form). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

Unlocking the Mystery of Third Party Reimbursement in Kinesiotherapy
Unlocking the Mystery of Third Party Reimbursement In Kinesiotherapy Provided by the American Kinesiotherapy Association Written by Joanne Byron, LPN, BSNH, CHA, CMC, CPC, MCMC, PCS Edited & Contributions made by Carl Byron, III, ATC-L, EMT-I, CHA, CPC, MCMC Health Care Consulting Services, Inc. PO Box 572 Medina, Ohio 44258-0572 www.hccsincorp.com Page 2 Table of Contents Page Introduction – Bridging the Gap between KT and Payor 3 Key Elements to Reimbursement 4 Provider Enrollment 5 State of Health Insurance 9 Medicare Benefit Policy Manual 10 Reimbursement Basics 12 Documentation 13 Appropriate Coding 14 Procedure Coding – a 2 Tier System 15 CPT Assistant 16 Diagnosis Code Assignment ICD-9-CM 21 ICD-10-CM 22 Understanding the Health Insurance Industry 26 Types of insurance and reimbursement methodology 26 Managed Care Contracting Considerations 30 Administrative Simplification 33 NCQA 37 Managed Care Terminology (terms & definitions from HHS) 38 Provided by the American Kinesiotherapy Association (AKTA) & Written by Health Care Consulting Services, Inc. (HCCS) Copyright © 2010 AKTA & HCCS, All Rights Reserved This document is not intended to provide legal or consulting advice. It is for educational purposes only. Please retain an attorney or consultant for specific information for your practice. Page 3 Introduction Bridging the gap between KT and payer “How do we get paid?” . A challenging question every health care provider faces, regardless of specialty and health insurance company. The purpose of this reimbursement manual is to provide a basic understanding of health care reimbursement and compliance prior to filing claims for Kinesiotherapy services. Physical medicine budgets are getting ever tighter, demands on rehab specialists’ time and skills are increasing. -

Clinical Vocabularies for Global Real World Evidence (RWE) | Evidera
Clinical Vocabularies for Global RWE Analysis Don O’Hara, MS Senior Research Associate, Real-World Evidence, Evidera Vernon F. Schabert, PhD Senior Research Scientist, Real-World Evidence, Evidera Introduction significant volume of real-world evidence (RWE) analyses continue to be conducted with data A repurposed from healthcare administrative Don O’Hara Vernon F. Schabert databases. The range of sources represented by those databases has grown in response to demand for richer description of patient health status and outcomes. information for improved quality and coordination Data availability, including the range of available data of care, often using standardized messaging systems sources, has grown unevenly across the globe in response such as Health Level 7 (HL7). These messages are to country-specific market and regulatory dynamics. only as good as the standardization of codes between Nonetheless, as demand globalizes for RWE insights from message senders and receivers, which motivates the databases, those demands increase pressure on analysts encoding of facts using common code sets. The second to find ways to bridge differences between local data is the increased availability of common data models to sources to achieve comparable insights across regions. standardize the extraction and analysis of these data for RWE and drug safety purposes. While common data One of the challenges in bridging differences across models make compromises on the structure of tables databases is the codes used to represent key clinical and fields extracted from healthcare systems such as facts. Historically, RWE database studies have leveraged electronic medical records (EMR) and billing systems, local code sets for cost-bearing healthcare services they can improve consistency and replicability of analyses such as drugs, procedures, and laboratory tests. -
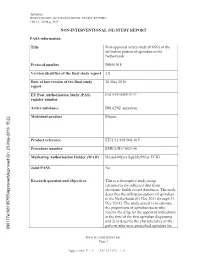
Non-Interventional (Ni) Study Report
Apixaban B0661018 NON-INTERVENTIONAL STUDY REPORT FINAL, 20 May 2015 NON-INTERVENTIONAL (NI) STUDY REPORT PASS information Title Post-approval safety study (PASS) of the utilization pattern of apixaban in the Netherlands Protocol number B0661018 Version identifier of the final study report 1.0 Date of last version of the final study 20 May 2016 report EU Post Authorisation Study (PAS) ENCEPP/SDPP/5177 register number Active substance B01AF02 apixaban Medicinal product Eliquis -2016 15:22 y Product reference EU/1/11/691/001-015 Procedure number EMEA/H/C/002148 Marketing Authorisation Holder (MAH) Bristol-Myers Squibb/Pfizer EEIG roved On: 23-Ma Joint PASS No pp Research question and objectives This is a descriptive study using roved\A retrospectively collected data from pp electronic health record databases. The study describes the utilization pattern of apixaban in the Netherlands (01 Dec 2011 through 31 Dec 2014). The study aimed 1) to estimate the proportions of apixaban users who receive the drug for the approved indications at the time of the first apixaban dispensing and 2) to describe the characteristics of the patients who were prescribed apixaban for 090177e188105755\A PFIZER CONFIDENTIAL Page 1 Approved v 1.0 930102376 1.0 Apixaban B0661018 NON-INTERVENTIONAL STUDY REPORT FINAL, 20 May 2015 on-label and off-label indications. Country(-ies) of study The Netherlands Author Marketing Authorisation Holder(s) Marketing Authorisation Holder(s) Bristol-Myers Squibb/Pfizer EEIG -2016 15:22 y MAH contact person roved On: 23-Ma pp roved\A pp This document contains confidential information belonging to Pfizer. -

Ata Lements for Mergency Epartment Ystems
D ata E lements for E mergency D epartment S ystems Release 1.0 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES CDCCENTERS FOR DISEASE CONTROL AND PREVENTION D ata E lements for E mergency D epartment S ystems Release 1.0 National Center for Injury Prevention and Control Atlanta, Georgia 1997 Data Elements for Emergency Department Systems, Release 1.0 (DEEDS) is the result of contributions by participants in the National Workshop on Emergency Department Data, held January 23-25, 1996, in Atlanta, Georgia, subsequent review and comment by individuals who read Release 1.0 in draft form, and finalization by a multidisciplinary writing committee. DEEDS is a set of recommendations published by the National Center for Injury Prevention and Control of the Centers for Disease Control and Prevention: Centers for Disease Control and Prevention David Satcher, MD, PhD, Director National Center for Injury Prevention and Control Mark L. Rosenberg, MD, MPP, Director Division of Acute Care, Rehabilitation Research, and Disability Prevention Richard J. Waxweiler, PhD, Director Acute Care Team Daniel A. Pollock, MD, Leader Production services were provided by the staff of the Office of Communication Resources, National Center for Injury Prevention and Control, and the Management Analysis and Services Office: Editing Valerie R. Johnson Cover Design and Layout Barbara B. Lord Use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services. This document and subsequent revisions can be found at the National Center for Injury Prevention and Control Web site: http://www.cdc.gov/ncipc/pub-res/deedspage.htm Suggested Citation: National Center for Injury Prevention and Control. -

Understanding Remittance Advice
Understanding the Remittance Advice: A Guide for Medicare Providers, Physicians, Suppliers, and Billers March 2006 DISCLAIMER This Guide was current at the time it was printed or downloaded. Medicare policy changes frequently so links to the source documents have been provided within the Guide for your reference. This Guide was prepared as a tool to assist providers and is not intended to grant rights or impose obligations. Although every reasonable effort has been made to assure the accuracy of the information within these pages, the ultimate responsibility for the correct submission of claims and response to any remittance advice lies with the provider of services. The Centers for Medicare & Medicaid Services (CMS) employees, agents, and staff make no representation, warranty, or guarantee that this compilation of Medicare information is error- free and will bear no responsibility or liability for the results or consequences of the use of this Guide. This publication is a general summary that explains certain aspects of the Medicare Program, but is not a legal document. The official Medicare Program provisions are contained in the relevant laws, regulations, and rulings. For ease of reading this Guide, the term “provider” is used generally to refer to providers of all Medicare Part A and Part B services, supplies, and equipment. When information specific to a particular provider type is presented, the name of the specific provider type is used [e.g., physician, supplier, hospital, Skilled Nursing Facility (SNF)]. Institutional providers refer to hospitals, SNFs, Home Health Agencies (HHAs), hospices, and other facility-based health care organizations that submit claims to Fiscal Intermediaries (FIs) and Regional Home Health Intermediaries (RHHIs). -

(Child Core Set) Technical Specifications and Reso
Core Set of Children's Health Care Quality Measures for Medicaid and CHIP (Child Core Set) Technical Specifications and Resource Manual for Federal Fiscal Year 2019 Reporting February 2019 Center for Medicaid and CHIP Services Centers for Medicare & Medicaid Services This page left blank for double-sided copying ii ACKNOWLEDGMENTS For Proprietary Codes: CPT® codes copyright 2018 American Medical Association. All rights reserved. CPT is a trademark of the American Medical Association. No fee schedules, basic units, relative values or related listings are included in CPT. The AMA assumes no liability for the data contained herein. Applicable FARS/DFARS restrictions apply to government use. SNOMED CLINICAL TERMS (SNOMED CT®) copyright 2004-2018 The International Health Terminology Standards Development Organisation. All Rights Reserved. The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) is published by the World Health Organization (WHO). ICD-9-CM is an official Health Insurance Portability and Accountability Act standard. The International Classification of Diseases, 9th Revision, Procedure Coding System (ICD-9-PCS) is published by the World Health Organization (WHO). ICD-9-PCS is an official Health Insurance Portability and Accountability Act standard. The International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) is published by the World Health Organization (WHO). ICD-10-CM is an official Health Insurance Portability and Accountability Act standard. The International Classification of Diseases, 10th Revision, Procedure Coding System (ICD-10-PCS) is published by the World Health Organization (WHO). ICD-10-PCS is an official Health Insurance Portability and Accountability Act standard. This material contains content from LOINC® (http://loinc.org). -

Inappropriate Primary Diagnosis Codes Policy, Professional
Commercial Reimbursement Policy CMS 1500 Policy Number 2020R0122C Inappropriate Primary Diagnosis Codes Policy, Professional IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY You are responsible for submission of accurate claims. This reimbursement policy is intended to ensure that you are reimbursed based on the code or codes that correctly describe the health care services provided. UnitedHealthcare reimbursement policies may use Current Procedural Terminology (CPT®*), Centers for Medicare and Medicaid Services (CMS) or other coding guidelines. References to CPT or other sources are for definitional purposes only and do not imply any right to reimbursement. This reimbursement policy applies to all health care services billed on CMS 1500 forms and, when specified, to those billed on UB04 forms. Coding methodology, industry-standard reimbursement logic, regulatory requirements, benefits design and other factors are considered in developing reimbursement policy. This information is intended to serve only as a general reference resource regarding UnitedHealthcare’s reimbursement policy for the services described and is not intended to address every aspect of a reimbursement situation. Accordingly, UnitedHealthcare may use reasonable discretion in interpreting and applying this policy to health care services provided in a particular case. Further, the policy does not address all issues related to reimbursement for health care services provided to UnitedHealthcare enrollees. Other factors affecting reimbursement may supplement, modify or, in some cases, supersede this policy. These factors may include, but are not limited to: legislative mandates, the physician or other provider contracts, the enrollee’s benefit coverage documents and/or other reimbursement, medical or drug policies. Finally, this policy may not be implemented exactly the same way on the different electronic claims processing systems used by UnitedHealthcare due to programming or other constraints; however, UnitedHealthcare strives to minimize these variations. -

G11.11: New Icd-10-Cm (Diagnosis) Code for Friedreich's Ataxia
G11.11: NEW ICD-10-CM (DIAGNOSIS) CODE FOR FRIEDREICH’S ATAXIA EFFECTIVE OCTOBER 1, 2020 CALL TO ACTION – PROVIDE THIS INFO TO YOUR CLINICIANS Starting on October 1, 2020, at your next appointments with any clinician, ask that your ICD-10 diagnosis code for FA be updated to G11.11 (previous code for FA was G11.1 > add a “1” > G11.11). Your clinician can use multiple ICD-10 diagnosis codes based on your need for medical care (e.g. G11.11 – FA, I49.9 - cardiac arrhythmia, Z13.1 – encounter for screening for diabetes mellitus). Please email [email protected] if you or your clinician has questions regarding use of G11.11 PREVIOUS CODING FOR FRIEDREICH’S ATAXIA ICD-9 system had a specific code for FA: 334.0 In conversion to ICD-10, the code for FA became less specific because it covered a group of conditions described as “early-onset cerebellar ataxias”. The previous FA ICD-10 diagnosis code was G11.1. BENEFITS OF THE NEW FA-SPECIFIC ICD-10-CM CODE G11.11 • Fewer rejected health insurance claims • Payer coverage determinations without need for direct access to patients’ medical records for review of medical necessity • Monitor adherence to clinical management guidelines and track care outcomes • Evaluate symptom progression of FA; additional ICD-10 codes can be applied to reflect context of FA diagnosis, such as for falls, surgeries, medications, and co-morbidities • Enhanced ability to track disease prevalence BACKGROUND ON ICD CODING SYSTEM ICD = International Classification of Diseases The ICD coding system was established by the World Health Organization; currently used by ~100 countries for reporting cause of death and epidemiology statistics. -

Month 2009 Issue
NOTE: Should you have landed here as a result of a search engine (or other) link, be advised that these files contain material that is copyrighted by the American Medical Association. You are forbidden to download the files unless you read, agree to, and abide by the provisions of the copyright statement. Read the copyright statement now and you will be linked back to here. April 2010 Issue In This Advisory Part A Articles Part A LCD Updates Part B Articles Part B LCD Updates Part A & B Articles Forms & Documents Acronyms Monthly Feature Questions and Answers on Reporting Physician Consultation Services This article is for physicians and non-physician practitioners (NPPs) who perform initial evaluation and management (E/M) services previously reported by Current Procedural Terminology (CPT) consultation codes for Medicare beneficiaries and submit claims to Medicare Carriers and/or Medicare Administrative Contractors (MACs) for those services. It is also intended for Method II critical access hospitals, which bill for the services of those physicians and NPPs. Continued on Page 36 www.palmettogba.com/J1a www.palmettogba.com/J1b www.cms.hhs.gov Table of Contents J1 Part A ......................................................................................................................................1 Accumulation of Claims with Condition Code 04 on the Provider Statistical and Reimbursement Report (PS and R)............... 1 April 2010 Update of the Hospital Outpatient Prospective Payment System (OPPS)...............................................................