2018 Annual Meeting Friday Handouts: Ultrasound 101
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Induction of Labor
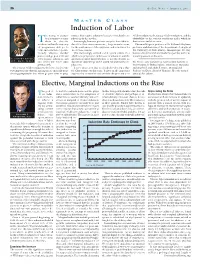
36 O B .GYN. NEWS • January 1, 2007 M ASTER C LASS Induction of Labor he timing of parturi- nancies that require induction because of medical com- of labor induction, the timing of labor induction, and the tion remains a conun- plications in the mother. advisability of the various conditions under which in- Tdrum in obstetric Increasingly, however, patients are apt to have labor in- duction can and does occur. medicine in that the majority duced for their own convenience, for personal reasons, This month’s guest professor is Dr. William F. Rayburn, of pregnancies will go to for the convenience of the physician, and sometimes for professor and chairman of the department of ob.gyn. at term and enter labor sponta- all of these reasons. the University of New Mexico, Albuquerque. Dr. Ray- neously, whereas another This increasingly utilized social option ushers in a burn is a maternal and fetal medicine specialist with a na- portion will go post term and whole new perspective on the issue of induction, and the tional reputation in this area. E. ALBERT REECE, often require induction, and question is raised about whether or not the elective in- M.D., PH.D., M.B.A. still others will enter labor duction of labor brings with it added risk and more com- DR. REECE, who specializes in maternal-fetal medicine, is prematurely. plications. Vice President for Medical Affairs, University of Maryland, The concept of labor induction, therefore, has become It is for this reason that we decided to develop a Mas- and the John Z. -

Review Article Umbilical Cord Hematoma: a Case Report and Review of the Literature
Hindawi Obstetrics and Gynecology International Volume 2018, Article ID 2610980, 6 pages https://doi.org/10.1155/2018/2610980 Review Article Umbilical Cord Hematoma: A Case Report and Review of the Literature Gennaro Scutiero,1 Bernardi Giulia,1 Piergiorgio Iannone ,1 Luigi Nappi,2 Danila Morano,1 and Pantaleo Greco1 1Department of Morphology, Surgery and Experimental Medicine, Section of Obstetrics and Gynecology, Azienda Ospedaliero-Universitaria S. Anna, University of Ferrara, Via Aldo Moro 8, 44121 Cona, Ferrara, Italy 2Department of Medical and Surgical Sciences, Institute of Obstetrics and Gynecology, University of Foggia, Viale L. Pinto, 71100 Foggia, Italy Correspondence should be addressed to Piergiorgio Iannone; [email protected] Received 17 December 2017; Accepted 21 February 2018; Published 26 March 2018 Academic Editor: John J. Moore Copyright © 2018 Gennaro Scutiero et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Objectives. To deepen the knowledge in obstetrics on a very rare pregnancy complication: umbilical cord hematoma. Methods.A review of the case reports described in the last ten years in the literature was conducted in order to evaluate epidemiology, predisposing factors, potential outcomes, prenatal diagnosis, and clinical management. Results. Spontaneous umbilical cord hematoma is a rare complication of pregnancy which represents a serious cause of fetal morbidity and mortality. *ere are many risk factors such as morphologic anomalies, infections, vessel wall abnormalities, iatrogenic causes, and traction or torsion of the cord, but the exact etiology is still unknown. -

15 Complications of Labor and Birth 279
Complications of 15 Labor and Birth CHAPTER CHAPTER http://evolve.elsevier.com/Leifer/maternity Objectives augmentation of labor (a˘wg-me˘n-TA¯ -shu˘n, p. •••) Bishop score (p. •••) On completion and mastery of Chapter 15, the student will be able to do cephalopelvic disproportion (CPD) (se˘f-a˘-lo¯ -PE˘L- v i˘c the following: di˘s-pro¯ -PO˘ R-shu˘n, p. •••) 1. Defi ne key terms listed. cesarean birth (se˘-ZA¯ R-e¯-a˘n, p. •••) 2. Discuss four factors associated with preterm labor. chorioamnionitis (ko¯ -re¯-o¯-a˘m-ne¯-o¯ -NI¯-ti˘s, p. •••) 3. Describe two major nursing assessments of a woman dysfunctional labor (p. •••) ˘ ¯ in preterm labor. dystocia (dis-TO-se¯-a˘, p. •••) episiotomy (e˘-pe¯ z-e¯-O˘ T- o¯ -me¯, p. •••) 4. Explain why tocolytic agents are used in preterm labor. external version (p. •••) 5. Interpret the term premature rupture of membranes. fern test (p. •••) 6. Identify two complications of premature rupture of forceps (p. •••) membranes. hydramnios (hi¯-DRA˘ M-ne¯-o˘s, p. •••) 7. Differentiate between hypotonic and hypertonic uterine hypertonic uterine dysfunction (hi¯-pe˘r-TO˘ N-i˘k U¯ -te˘r-i˘n, dysfunction. p. •••) 8. Name and describe the three different types of breech hypotonic uterine dysfunction (hi¯-po¯-TO˘ N-i˘k, p. •••) presentation. induction of labor (p. •••) ¯ 9. List two potential complications of a breech birth. multifetal pregnancy (mu˘l-te¯-FE-ta˘l, p. •••) nitrazine paper test (NI¯-tra˘-ze¯n, p. •••) 10. Explain the term cephalopelvic disproportion (CPD), and oligohydramnios (p. •••) discuss the nursing management of CPD. -

Induction of Labour in Late and Postterm Pregnancies and Its
Original Article 793 Induction of Labour in Late and Postterm Pregnancies and its Impact on Maternal and Neonatal Outcome Die Geburtseinleitung bei übertragener Schwangerschaft und die Auswirkungen auf mütterliches und kindliches Outcome Authors F. Thangarajah*, P. Scheufen*, V. Kirn, P. Mallmann Affiliation University Hospital of Cologne, Department of Obstetrics and Gynecology, Cologne, Germany Key words Abstract Zusammenfassung l" induction of labour ! ! l" delivery Introduction: This study aimed to determine the Einleitung: Diese Studie untersuchte die Auswir- l" cesarean section effects of induction of labour in late-term preg- kungen der Geburtseinleitung in der Spätschwan- l" materno‑fetal medicine nancies on the mode of delivery, maternal and gerschaft bzw. bei Übertragung auf die Art der Schlüsselwörter neonatal outcome. Entbindung sowie auf das mütterliche und kind- l" Geburtseinleitung Methods: We retrospectively analyzed deliveries liche Outcome. l" Entbindung between 2000 and 2014 at the University Hospi- Methoden: Alle in der Universitätsklinik Köln l" Kaiserschnittentbindung tal of Cologne. Women with a pregnancy aged be- zwischen 2000 und 2014 erfolgten Entbindungen l" Perinatalmedizin tween 41 + 0 to 42 + 6 weeks were included. wurden retrospektiv untersucht. Alle Frauen, die Those who underwent induction of labour were in der 41 + 0 bis 42 + 6 Schwangerschaftswoche compared with women who were expectantly entbanden, wurden in die Studie eingeschlossen. managed. Maternal and neonatal outcomes were Schwangere Frauen, bei denen eine Geburtsein- evaluated. leitung durchgeführt wurde, wurden mit Frauen Results: 856 patients were included into the verglichen, die exspektativ behandelt wurden. study. The rate of cesarean deliveries was signifi- Die mütterlichen und kindlichen Outcomes wur- cantly higher for the induction of labour group den ausgewertet. -

Induction of Labour at Term in Older Mothers
Induction of Labour at Term in Older Mothers Scientific Impact Paper No. 34 February 2013 Induction of Labour at Term in Older Mothers 1. Background and introduction The average age of childbirth is rising markedly across Western countries.1 In the United Kingdom (UK) the proportion of maternities in women aged 35 years or over has increased from 8% (approximately 180 000 maternities) in 1985–87 to 20% (almost 460 000 maternities) in 2006–8 and in women aged 40 years and older has trebled in this time from 1.2% (almost 27 000 maternities) to 3.6% (approximately 82 000 maternities).2 There is a continuum of risk for both mother and baby with rising maternal age with numerous studies reporting multiple adverse fetal and maternal outcomes associated with advanced maternal age. Obstetric complications including placental abruption,3 placenta praevia, malpresentation, low birthweight,4–7 preterm8 and post–term delivery9 and postpartum haemorrhage,10 are higher in older mothers. As fertility declines with age, there is a greater use of assisted reproductive technologies (ARTs) and the possibility of multiple pregnancy increases. This may independently adversely affect the risks reported.11 Preexisting maternal medical conditions including hypertension, obesity and diabetes increase with advancing maternal age as do pregnancy–related maternal complications such as pre–eclampsia and gestational diabetes.12 These medical co–morbidities can all influence fetal health and are likely to compound the effect of age on the risk of pregnancy in an older -

A Systematic Approach to Stillbirth Examination in a Tertiary Hospital
ORIGINAL ARTICLE A Systematic Approach to Stillbirth Examination in a Tertiary Hospital Arby Jane R. Igualada,1 Efren J. Domingo2 and Jose Maria C. Avila3 1Department of Obstetrics and Gynecology, Philippine General Hospital, University of the Philippines Manila 2Department of Obstetrics and Gynecology, College of Medicine and Philippine General Hospital, University of the Philippines Manila 3Department of Pathology, College of Medicine, University of the Philippines Manila ABSTRACT Background. Stillbirth has a complex pathophysiology, hence the difficulty in arriving at a specific cause. Objectives. The study aimed to identify the probable causes of stillbirth in a tertiary hospital based on gross examination of the placenta and the fetus, as well as, to identify the demographic profile of the stillbirths. Methods. A cross-sectional descriptive study was conducted among 29 stillbirths delivered in a tertiary hospital from March 2016 to September 2016. The probable causes of stillbirth were categorized as obstetrics complications, placental abnormalities, umbilical cord abnormalities, fetal malformations, infections, hypertensive disorders, medical complications, and undetermined causes. Results. 86% of stillbirths in this study had a probable cause of death. Umbilical and placental abnormalities were the most probable causes (62% and 41%, respectively). The two most common identified cord abnormalities were short cord length (34%) and marginal insertion (23%), while small placenta (27%) was the most common for placental abnormalities. Conclusion. To be able to come up with the probable cause of stillbirth, the delivering physician or health personnel should always account the gross findings of the fetus and placenta after delivery. Key Words: fetal death in utero, placenta, stillbirth evaluation INTRODUCTION Obstetricians and other delivering clinicians should contribute to providing significant findings during fetal and placental examination of stillbirths. -

Complications of Delivery Guy Peifer
Complications of Delivery Guy Peifer • Childbirth is usually a happy event. • Usually occurs without worry. • But…..occasionally something goes wrong. Posterior (Sunny Side Up) Frank Breech Complete Breech Footling Breech Transverse Twins The female reproductive system includes two ovaries, two fallopian tubes, the uterus, and the vagina. • The umbilical cord connects the fetus and placenta. • The umbilical vein carries blood to the fetus. • The umbilical arteries carry blood to the placenta. Fetal Development • The amniotic sac encloses the fetus in amniotic fluid. • The fourth through eighth week of embryonic development are critical. • Major organs and other body systems are most susceptible to damage as they form. Fetal Development • Gestational period: time it takes the fetus to develop in utero • Normally 38 weeks • Calculated from the first day of the pregnant woman’s last menstrual period Supine Hypotensive Syndrome • Sensitivity to body position increases as gestation increases. • Lying supine can cause compression of the inferior vena cava. • If pressure is not relieved, cardiac output is decreased. Special Terminology • : • Gravidity—number of times pregnant • Parity—delivery of an infant who is alive • Primigravida—woman pregnant for first time • Primipara—woman with only one delivery • Multigravida—two or more pregnancies Special Terminology • Multipara—two or more deliveries • Grand multipara—more than five deliveries • Nullipara—never delivered Primary Assessment • Transport decision • Provide rapid transport for patients: • With significant bleeding and pain • Who are hypertensive • Who are having a seizure • Who have an altered mental status History Taking • Determine chief complaint using OPQRSTI. • Obtain the SAMPLE history. • Determine estimated due date. • Determine previous complications or gynecologic problems. -

Late-Term and Postterm Pregnancy
Common Questions About Late-Term and Postterm Pregnancy MARY WANG, MD, University of California, San Diego, California PATRICIA FONTAINE, MD, MS, HealthPartners Institute for Education and Research, Bloomington, Minnesota Pregnancy is considered late term from 41 weeks, 0 days’ to 41 weeks, 6 days’ gestation, and postterm at 42 weeks’ gestation. Early dating of the pregnancy is important for accurately determining when a pregnancy is late- or postterm, and first-trimester ultrasonography should be performed if clinical dating is uncertain. Optimal management of a low-risk, late-term preg- nancy should consider maternal preference and balance the benefits and risks of induction vs. waiting for spontaneous labor. Compared with expectant management, induction at 41 weeks’ gestation is associated with a small absolute decrease in perinatal mortality and decreases in other fetal and maternal risks without an increased risk of cesarean delivery. Although there is no clear evidence that antenatal testing beginning at 41 weeks’ gestation prevents intrauterine fetal demise, it is often performed because the risks are low. When expectant management is chosen, most experts recommend beginning twice-weekly antenatal surveillance at 41 weeks with biophysical profile or nonstress testing plus amniotic fluid index (modified biophysical profile); induction may be deferred until 42 weeks if this surveillance is reassuring. Am( Fam Physician. 2014;90(3):160-165. Copyright © 2014 American Academy of Family Physicians.) CME This clinical content ostterm pregnancy is defined as pregnancy has been demonstrated.5-7 A conforms to AAFP criteria that lasting beyond 294 days or 42 woman who was born postterm has a 49% for continuing medical education (CME). -

AOM Clinical Practice Guideline – Pregnancy Beyond 41 Weeks
CLINICAL PRACTICE GUIDELINE10 Management of the UNCOMPLICATED PREGNANCY BEYOND 41+0 WEEKS GESTATION February 2010 Clinical Practice Guideline No.10 Management of the UNCOMPLICATED PREGNANCY BEYOND 41+0 WEEKS’ GESTATION AUTHORS AOM STAFF Julie Corey, RM MHSc Suzannah Bennett, MHSc Tasha MacDonald, RM MHSc Cindy Hutchinson, MSc Bobbi Soderstrom, RM CONTRIBUTORS Clinical Practice Guideline Subcommittee ACKNOWLEDGEMENTS Elizabeth Darling, RM MSc, Chair Kristen Dennis, RM Cheryllee Bourgeois, RM Ontario Ministry of Health and Long-term Care Corinne Hare, RM Ryerson University Midwifery Education Program Jenni Huntly, RM Paula Salehi, RM The Association of Ontario Midwives respectfully Lynlee Spencer, BSc acknowledges the financial support of the Ministry of Vicki Van Wagner, RM, PhD (c) Health and Long-Term Care in the development of this Rhea Wilson, RM guideline. INSURANCE AND RISK MANAGEMENT The views expressed in this guideline are strictly those PROGRAM STEERING COMMITTEE of the Association of Ontario Midwives. No official ‘Remi Ejiwunmi, RM, Chair endorsement by the Ministry of Health and Long-Term Abigail Corbin, RM Care is intended or should be inferred. Elana Johnston, RM Carolynn Prior van Fraassen, RM Lisa M Weston, RM This document may be cited as: Postdates CPG Working Group. Association of Ontario Midwives. Management of the Uncomplicated Pregnancy Beyond 41+0 Weeks’ Gestation. February 2010. The AOM is committed, through our statement on Gender Inclusivity and Human Rights, to reflect and include trans, genderqueer and intersex communities in all aspects of our work.In this document, there are references to sources that use gendered language to refer to populations of pregnant and birthing people. -

Stillbirth Research and Education Submission
To the Committee on Stillbirth Inquiry: Umbilical Cord Pathology is a significant cause of stillbirth . Stillbirth Prevention requires addressing UCA which can be imaged and managed. Dear Senator Keneally, Your initiation of "Inquiry into stillbirth in Austrailia" caught my attention. Buzz Feed's story of Ms Imrie and yours are not uncommon. Stillbirth can be prevented and It would be a privileged to assist you in your endeavor. We observed that stillbirth occurs during maternal sleep .This has been published and later confirmed by several studies including one from NZ. The observation means stillbirth is at a definate time and not random. This means it can be prevented. We have used Home Fetal Monitoring for 25 years to address this tendancy. Please let me know if you would like to discuss this approach. Regards, Jason H Collins MD,MSCR 1566 Letters December 1997 .aan .] Obstet Gynecol lation of a radiopaque contrast medium (Thorotrast) accidents. Over a 1-year period >2000 "hits" have been after intraamniotic injection. Because delivery is likely recorded. These inquiries led to 20 consecutive inter- to occur in a short period of time, the degree of views from around the United States. Questions per- therapy that could be provided may be limited. Finally, tained to the events surrounding stillbirth caused by an intraamniotic fetal lung therapy may be more effective umbilical cord accident. Mothers seemed to readily if the therapeutic agent is delivered before the onset of remember the details of fetal behavior and all recalled labor at a time when the fetus breaths more actively fetal movement the day before death. -

Postdated Pregnancy: Its Maternal and Fetal Outcome
International Journal of Reproduction, Contraception, Obstetrics and Gynecology Singh N et al. Int J Reprod Contracept Obstet Gynecol. 2020 Aug;9(8):3223-3227 www.ijrcog.org pISSN 2320-1770 | eISSN 2320-1789 DOI: http://dx.doi.org/10.18203/2320-1770.ijrcog20203299 Original Research Article Postdated pregnancy: its maternal and fetal outcome Neetu Singh*, Devyani Misra, Shubhi Srivastava Department of Obstetrics and Gynecology, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India Received: 18 May 2020 Accepted: 29 June 2020 *Correspondence: Dr. Neetu Singh, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Postdated pregnancy is one of the commonest obstetric conditions. Pregnancy is called term when it lies between 37 weeks to 42 weeks from the last menstrual period. If the pregnancy exceeds 40 weeks it is called as postdated pregnancy. The overall incidence of post term pregnancy is 7% of all pregnancies. Methods: This observational study was carried out in the department of obstetrics and gynecology in Dr Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India from September 2019 to February 2020. Total 100 postdated pregnancy enrolled in the study those willing to participate and fulfilling the inclusion and exclusion criteria. Aim is to assess maternal and fetal outcome in postdated pregnancy. Results: In present study, incidence of postdated pregnancy was found to be 5% and number of normal deliveries was 66 (66%), LSCS were 32 (32%) and 2 (2%) were instrumental delivery. -

Increased Placental Cell Senescence and Oxidative Stress in Women with Pre-Eclampsia and Normotensive Post-Term Pregnancies
International Journal of Molecular Sciences Article Increased Placental Cell Senescence and Oxidative Stress in Women with Pre-Eclampsia and Normotensive Post-Term Pregnancies Paula J. Scaife 1, Amy Simpson 2, Lesia O. Kurlak 3, Louise V. Briggs 4, David S. Gardner 3, Fiona Broughton Pipkin 2, Carolyn J. P. Jones 5 and Hiten D. Mistry 6,* 1 Clinical, Metabolic and Molecular Physiology Research Group, University of Nottingham, Nottingham NG7 2RD, UK; [email protected] 2 Department of Obstetrics & Gynaecology, University of Nottingham, Nottingham NG7 2RD, UK; [email protected] (A.S.); [email protected] (F.B.P.) 3 School of Veterinary Medicine and Science, University of Nottingham, Nottingham NG7 2RD, UK; [email protected] (L.O.K.); [email protected] (D.S.G.) 4 School of Engineering, University of Nottingham, Nottingham NG7 2RD, UK; [email protected] 5 Maternal & Fetal Health Research Centre, Manchester Academic Health Science Centre, University of Manchester, Manchester M13 9PL, UK; [email protected] 6 Department of Women and Children’s Health, School of Life Course Sciences, King’s College London, London SE5 9NU, UK * Correspondence: [email protected] Citation: Scaife, P.J.; Simpson, A.; Abstract: Up to 11% of pregnancies extend to post-term with adverse obstetric events linked to Kurlak, L.O.; Briggs, L.V.; Gardner, pregnancies over 42 weeks. Oxidative stress and senescence (cells stop growing and dividing by D.S.; Broughton Pipkin, F.; Jones, irreversibly arresting their cell cycle and gradually ageing) can result in diminished cell function.