Induction of Labour at Term in Older Mothers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Induction of Labor
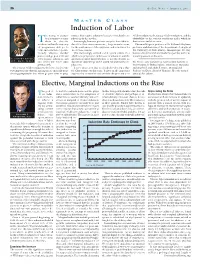
36 O B .GYN. NEWS • January 1, 2007 M ASTER C LASS Induction of Labor he timing of parturi- nancies that require induction because of medical com- of labor induction, the timing of labor induction, and the tion remains a conun- plications in the mother. advisability of the various conditions under which in- Tdrum in obstetric Increasingly, however, patients are apt to have labor in- duction can and does occur. medicine in that the majority duced for their own convenience, for personal reasons, This month’s guest professor is Dr. William F. Rayburn, of pregnancies will go to for the convenience of the physician, and sometimes for professor and chairman of the department of ob.gyn. at term and enter labor sponta- all of these reasons. the University of New Mexico, Albuquerque. Dr. Ray- neously, whereas another This increasingly utilized social option ushers in a burn is a maternal and fetal medicine specialist with a na- portion will go post term and whole new perspective on the issue of induction, and the tional reputation in this area. E. ALBERT REECE, often require induction, and question is raised about whether or not the elective in- M.D., PH.D., M.B.A. still others will enter labor duction of labor brings with it added risk and more com- DR. REECE, who specializes in maternal-fetal medicine, is prematurely. plications. Vice President for Medical Affairs, University of Maryland, The concept of labor induction, therefore, has become It is for this reason that we decided to develop a Mas- and the John Z. -

Placenta Praevia/Low-Lying Placenta
LOCAL OPERATING PROCEDURE – CLINICAL Approved Quality & Patient Safety Committee 18/6/20 Review June 2022 PLACENTA PRAEVIA/LOW-LYING PLACENTA This LOP is developed to guide clinical practice at the Royal Hospital for Women. Individual patient circumstances may mean that practice diverges from this LOP. 1. AIM • Diagnosis and clinical management of woman with low-lying placenta (LLP) or placenta praevia (PP) 2. PATIENT • Pregnant woman with a LLP/PP after 20 weeks gestation 3. STAFF • Medical and midwifery staff 4. EQUIPMENT • Ultrasound • 16-gauge intravenous (IV) cannula • Blood tubes 5. CLINICAL PRACTICE Screening: • Recommend a morphology ultrasound at 18-20 weeks gestation to ascertain placental location • Include, on the ultrasound request form, any history of uterine surgery e.g. previous caesarean section (CS), myomectomy, to ensure that features of placenta accreta are examined • Identify woman with a: o LLP i.e. within 2cm of, but not covering the internal cervical os o PP i.e. covering the internal cervical os Antenatal care: • Reassure woman that 9 out of 10 LLP found at 18-20 week morphology ultrasound are no longer low-lying by term1 • Reassure woman with LLP that if she remains asymptomatic, there is no increased risk of adverse outcomes in the mid-trimester and she can continue normal activities e.g. travel, intercourse, exercise2 • Advise woman with LLP from 36 weeks that it is not a contraindication for trial of labour, chance of vaginal birth approximately9: o 43% if placenta is 0-10mm from cervical os o 85% if placenta -

Cohort Study of High Maternal Body Mass Index and the Risk of Adverse Pregnancy and Delivery Outcomes in Scotland
Open access Original research BMJ Open: first published as 10.1136/bmjopen-2018-026168 on 20 February 2020. Downloaded from Cohort study of high maternal body mass index and the risk of adverse pregnancy and delivery outcomes in Scotland Lawrence Doi ,1 Andrew James Williams ,2 Louise Marryat ,3,4 John Frank3,5 To cite: Doi L, Williams AJ, ABSTRACT Strengths and limitations of this study Marryat L, et al. Cohort Objective To examine the association between high study of high maternal maternal weight status and complications during pregnancy ► This study used a large, retrospectively accessed body mass index and the and delivery. risk of adverse pregnancy but cohort- structured, national database covering Setting Scotland. and delivery outcomes some of the major maternal and neonatal outcomes Participants Data from 132 899 first- time singleton in Scotland. BMJ Open in Scotland over eight recent years. deliveries in Scotland between 2008 and 2015 were used. 2020;10:e026168. doi:10.1136/ ► Analyses were adjusted for some of the key potential Women with overweight and obesity were compared with bmjopen-2018-026168 confounders to estimate impact of high maternal- women with normal weight. Associations between maternal ► Prepublication history and weight status on each outcome. body mass index and complications during pregnancy and 2 additional material for this ► All women with body mass index (BMI) of 30 kg/m delivery were evaluated. paper are available online. To or more were considered as having obesity; it is like- Outcome measures Gestational diabetes, gestational view these files, please visit ly that differentiating morbid obesity or obesity class hypertension, pre- eclampsia, placenta praevia, placental the journal online (http:// dx. -

Review Article Umbilical Cord Hematoma: a Case Report and Review of the Literature
Hindawi Obstetrics and Gynecology International Volume 2018, Article ID 2610980, 6 pages https://doi.org/10.1155/2018/2610980 Review Article Umbilical Cord Hematoma: A Case Report and Review of the Literature Gennaro Scutiero,1 Bernardi Giulia,1 Piergiorgio Iannone ,1 Luigi Nappi,2 Danila Morano,1 and Pantaleo Greco1 1Department of Morphology, Surgery and Experimental Medicine, Section of Obstetrics and Gynecology, Azienda Ospedaliero-Universitaria S. Anna, University of Ferrara, Via Aldo Moro 8, 44121 Cona, Ferrara, Italy 2Department of Medical and Surgical Sciences, Institute of Obstetrics and Gynecology, University of Foggia, Viale L. Pinto, 71100 Foggia, Italy Correspondence should be addressed to Piergiorgio Iannone; [email protected] Received 17 December 2017; Accepted 21 February 2018; Published 26 March 2018 Academic Editor: John J. Moore Copyright © 2018 Gennaro Scutiero et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Objectives. To deepen the knowledge in obstetrics on a very rare pregnancy complication: umbilical cord hematoma. Methods.A review of the case reports described in the last ten years in the literature was conducted in order to evaluate epidemiology, predisposing factors, potential outcomes, prenatal diagnosis, and clinical management. Results. Spontaneous umbilical cord hematoma is a rare complication of pregnancy which represents a serious cause of fetal morbidity and mortality. *ere are many risk factors such as morphologic anomalies, infections, vessel wall abnormalities, iatrogenic causes, and traction or torsion of the cord, but the exact etiology is still unknown. -

15 Complications of Labor and Birth 279
Complications of 15 Labor and Birth CHAPTER CHAPTER http://evolve.elsevier.com/Leifer/maternity Objectives augmentation of labor (a˘wg-me˘n-TA¯ -shu˘n, p. •••) Bishop score (p. •••) On completion and mastery of Chapter 15, the student will be able to do cephalopelvic disproportion (CPD) (se˘f-a˘-lo¯ -PE˘L- v i˘c the following: di˘s-pro¯ -PO˘ R-shu˘n, p. •••) 1. Defi ne key terms listed. cesarean birth (se˘-ZA¯ R-e¯-a˘n, p. •••) 2. Discuss four factors associated with preterm labor. chorioamnionitis (ko¯ -re¯-o¯-a˘m-ne¯-o¯ -NI¯-ti˘s, p. •••) 3. Describe two major nursing assessments of a woman dysfunctional labor (p. •••) ˘ ¯ in preterm labor. dystocia (dis-TO-se¯-a˘, p. •••) episiotomy (e˘-pe¯ z-e¯-O˘ T- o¯ -me¯, p. •••) 4. Explain why tocolytic agents are used in preterm labor. external version (p. •••) 5. Interpret the term premature rupture of membranes. fern test (p. •••) 6. Identify two complications of premature rupture of forceps (p. •••) membranes. hydramnios (hi¯-DRA˘ M-ne¯-o˘s, p. •••) 7. Differentiate between hypotonic and hypertonic uterine hypertonic uterine dysfunction (hi¯-pe˘r-TO˘ N-i˘k U¯ -te˘r-i˘n, dysfunction. p. •••) 8. Name and describe the three different types of breech hypotonic uterine dysfunction (hi¯-po¯-TO˘ N-i˘k, p. •••) presentation. induction of labor (p. •••) ¯ 9. List two potential complications of a breech birth. multifetal pregnancy (mu˘l-te¯-FE-ta˘l, p. •••) nitrazine paper test (NI¯-tra˘-ze¯n, p. •••) 10. Explain the term cephalopelvic disproportion (CPD), and oligohydramnios (p. •••) discuss the nursing management of CPD. -

Umbilical Cord Prolapse Guideline
Umbilical Cord Prolapse Guideline Document Control Title Umbilical Cord Prolapse Guideline Author Author’s job title Specialty Trainee in Obstetrics and Gynaecology Directorate Department Women’s and Children’s Obstetrics and Gynaecology Date Version Status Comment / Changes / Approval Issued 1.0 Mar Final Approved by the Maternity Services Guideline Group in 2011 April 2011. 1.1 Aug Revision Minor amendments by Corporate Governance to 2012 document control report, headers and footers, new table of contents, formatting for document map navigation. 2.0 Feb Final Approved by the Maternity Services Guideline Group in 2016 February 2016. 2.1 Apr Revision Harmonised with Royal Devon & Exeter guideline 2019 3.0 May Final Approved by Maternity Specialist Governance Forum 2019 meeting on 01.05.2019 Main Contact ST1 O&G Tel: Direct Dial– 01271 311806 North Devon District Hospital Raleigh Park Barnstaple Devon EX31 4JB Lead Director Medical Director Superseded Documents Nil Issue Date Review Date Review Cycle May 2019 May 2022 Three years Consulted with the following stakeholders: (list all) Senior obstetricians Senior midwives Senior management team Filename Umbilical Cord Prolapse Guideline v3. 01May 19.doc Policy categories for Trust’s internal Tags for Trust’s internal website (Bob) website (Bob) Cord, accidents, prolapse Maternity Services Maternity Page 1 of 11 Umbilical Cord Prolapse Guideline CONTENTS Document Control .................................................................................................... 1 1. Introduction -

Low-Lying Placenta
LOW- LYING PLACENTA LOW-LYING PLACENTA WHAT IS PLACENTA PRAEVIA? The placenta develops along with the baby in the uterus (womb) during pregnancy. It connects the baby with the mother’s blood system and provides the baby with its source of oxygen and nourishment. The placenta is delivered after the baby and is also called the afterbirth. In some women the placenta attaches low in the uterus and may be near, or cover a part, or lie over the cervix (entrance to the womb). If it is shown in early ultrasound scans, it is called a low-lying placenta. In most cases, the placenta moves upwards as the uterus enlarges. For some women the placenta continues to lie in the lower part of the uterus in the last months of pregnancy. This condition is known as placenta praevia. If the placenta covers the cervix, this is known as major placenta praevia. Normal Placenta Placenta Praevia Major Placenta Praevia WHAT ARE THE RISKS TO MY BABY AND ME? When the placenta is in the lower part of the womb, there is a risk that you may bleed in the second half of pregnancy. Bleeding from placenta praevia can be heavy, and so put the life of the mother and baby at risk. However, deaths from placenta praevia are rare. You are more likely to need a caesarean section because the placenta is in the way of your baby being born. HOW IS PLACENTA PRAEVIA DIAGNOSED? A low-lying placenta may be suspected during the routine 20-week ultrasound scan. Most women who have a low-lying placenta at the routine 20-week scan will not go on to have a low-lying placenta later in the pregnancy – only 1 in 10 go on to have a placenta praevia. -

Induction of Labour in Late and Postterm Pregnancies and Its
Original Article 793 Induction of Labour in Late and Postterm Pregnancies and its Impact on Maternal and Neonatal Outcome Die Geburtseinleitung bei übertragener Schwangerschaft und die Auswirkungen auf mütterliches und kindliches Outcome Authors F. Thangarajah*, P. Scheufen*, V. Kirn, P. Mallmann Affiliation University Hospital of Cologne, Department of Obstetrics and Gynecology, Cologne, Germany Key words Abstract Zusammenfassung l" induction of labour ! ! l" delivery Introduction: This study aimed to determine the Einleitung: Diese Studie untersuchte die Auswir- l" cesarean section effects of induction of labour in late-term preg- kungen der Geburtseinleitung in der Spätschwan- l" materno‑fetal medicine nancies on the mode of delivery, maternal and gerschaft bzw. bei Übertragung auf die Art der Schlüsselwörter neonatal outcome. Entbindung sowie auf das mütterliche und kind- l" Geburtseinleitung Methods: We retrospectively analyzed deliveries liche Outcome. l" Entbindung between 2000 and 2014 at the University Hospi- Methoden: Alle in der Universitätsklinik Köln l" Kaiserschnittentbindung tal of Cologne. Women with a pregnancy aged be- zwischen 2000 und 2014 erfolgten Entbindungen l" Perinatalmedizin tween 41 + 0 to 42 + 6 weeks were included. wurden retrospektiv untersucht. Alle Frauen, die Those who underwent induction of labour were in der 41 + 0 bis 42 + 6 Schwangerschaftswoche compared with women who were expectantly entbanden, wurden in die Studie eingeschlossen. managed. Maternal and neonatal outcomes were Schwangere Frauen, bei denen eine Geburtsein- evaluated. leitung durchgeführt wurde, wurden mit Frauen Results: 856 patients were included into the verglichen, die exspektativ behandelt wurden. study. The rate of cesarean deliveries was signifi- Die mütterlichen und kindlichen Outcomes wur- cantly higher for the induction of labour group den ausgewertet. -

Induction Indication: Advanced Maternal Age
INDUCTION INDICATION: ADVANCED MATERNAL AGE DOCUMENT TYPE: REFERENCE TOOL Level of Evidence INDUCTION INDICATION: Maternal Age ≥ 40 years RECOMMENDATION: Offer Induction at ≥ 39 weeks gestation Recommendation for labour induction: II-B Recommendation for timing of labour induction: II-B Recommendations for priority: II-B Definition The average age of childbirth is rising in developed countries. The definition of advanced maternal age for the purposes of this document is greater than or equal to 40 years of age. Studies report that advanced maternal age is associated with increased pregnancy risk for both mother and baby. Although the incidence of stillbirth at term is low, it is higher in women of advanced maternal age.1 Outcomes There are known adverse perinatal outcomes for women who are of advanced maternal age. When compared to younger women, in older women there is increased risk for placental abruption, placenta previa, malpresentation, growth restriction, preterm and postdates delivery as well as postpartum hemorrhage. Even after controlling for medical co-morbidities, advanced maternal age is found to be associated with an increase in antenatal and intrapartum stillbirth.2 The relative risk of stillbirth for women ≥ 40 years of age, for both multiparous and primiparous women is 1.2 to 4.5. The cumulative risk of stillbirth in women 40 and older at 39 weeks gestation is similar to mothers 25 to 29 years at 41 weeks gestation3,4. The mechanism for increased risk of stillbirth in older mothers is as yet unknown. Recommendations Labour induction may be offered to mothers who are greater than or equal to 40 years of age at 39 weeks of gestation. -

Antepartum Haemorrhage
OBSTETRICS AND GYNAECOLOGY CLINICAL PRACTICE GUIDELINE Antepartum haemorrhage Scope (Staff): WNHS Obstetrics and Gynaecology Directorate staff Scope (Area): Obstetrics and Gynaecology Directorate clinical areas at KEMH, OPH and home visiting (e.g. Community Midwifery Program) This document should be read in conjunction with this Disclaimer Contents Initial management: MFAU APH QRG ................................................. 2 Subsequent management of APH: QRG ............................................. 5 Management of an APH ........................................................................ 7 Key points ............................................................................................................... 7 Background information .......................................................................................... 7 Causes of APH ....................................................................................................... 7 Defining the severity of an APH .............................................................................. 8 Initial assessment ................................................................................................... 8 Emergency management ........................................................................................ 9 Maternal well-being ................................................................................................. 9 History taking ....................................................................................................... -

Oocyte Or Embryo Donation to Women of Advanced Reproductive Age: an Ethics Committee Opinion
ASRM PAGES Oocyte or embryo donation to women of advanced reproductive age: an Ethics Committee opinion Ethics Committee of the American Society for Reproductive Medicine American Society for Reproductive Medicine, Birmingham, Alabama Advanced reproductive age (ARA) is a risk factor for female infertility, pregnancy loss, fetal anomalies, stillbirth, and obstetric com- plications. Oocyte donation reverses the age-related decline in implantation and birth rates of women in their 40s and 50s and restores pregnancy potential beyond menopause. However, obstetrical complications in older patients remain high, particularly related to oper- ative delivery and hypertensive and cardiovascular risks. Physicians should perform a thorough medical evaluation designed to assess the physical fitness of a patient for pregnancy before deciding to attempt transfer of embryos to any woman of advanced reproductive age (>45 years). Embryo transfer should be strongly discouraged or denied to women of ARA with underlying conditions that increase or exacerbate obstetrical risks. Because of concerns related to the high-risk nature of pregnancy, as well as longevity, treatment of women over the age of 55 should generally be discouraged. This statement replaces the earlier ASRM Ethics Committee document of the same name, last published in 2013 (Fertil Steril 2013;100:337–40). (Fertil SterilÒ 2016;106:e3–7. Ó2016 by American Society for Reproductive Medicine.) Key Words: Ethics, third-party reproduction, complications, pregnancy, parenting Discuss: You can discuss -

Pregnancy After Age 35
REFLECTING ON THE TREND: Pregnancy After Age 35 A guide to Advanced Maternal Age for Ontario service providers, including a summary of statistical trends, influencing factors, health benefits, health risks and recommendations for care. A collaborative project of: Best Start: Ontario’s Maternal, Newborn and Early Child Development Resource Centre and the Halton Region Health Department 2007 ACKNOWLEDGEMENTS This Best Start Resource Centre manual was developed in collaboration with the Halton Region Health Department. The Best Start Resource Centre would like to thank Halton Region’s Health Department for entering into this partnership, and Kathryn Bamford, Reproductive Health Manager, for her persistence in determining a strategy to make this happen. The Halton Region Health Department is located west of Toronto and is responsible for public health in the communities of Burlington, Halton Hills, Milton and Oakville. Halton region has one of the highest rates of pregnancy over the age of 35 in Ontario, and is very interested in strategies for care of this population. For more information on the Halton Region Health Department, call 905-825-6000 or visit www.halton.ca/health The Best Start Resource Centre would like to thank Michelle Schwarz, BScN, MPA, Public Health Nurse, at the Halton Region Health Department for her work in researching and drafting this publication. Best Start would also like to Key Informants and • Joyce Engel, PhD, • Hana Sroka, MSc, CCGC, acknowledge the important Expert Reviewers: Vice-President, Academic Genetic Counsellor, Mount Niagara College Sinai Hospital roles played by the following • Dr. Sean Blaine, BSc, MD Halton Region staff: CCFP, Lead Physician, STAR • Dr.