Title Ecology of Long-Tailed Macaques (Macaca
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Called “Talking Animals” Taught Us About Human Language?
Linguistic Frontiers • 1(1) • 14-38 • 2018 DOI: 10.2478/lf-2018-0005 Linguistic Frontiers Representational Systems in Zoosemiotics and Anthroposemiotics Part I: What Have the So- Called “Talking Animals” Taught Us about Human Language? Research Article Vilém Uhlíř* Theoretical and Evolutionary Biology, Department of Philosophy and History of Sciences. Charles University. Viničná 7, 12843 Praha 2, Czech Republic Received ???, 2018; Accepted ???, 2018 Abstract: This paper offers a brief critical review of some of the so-called “Talking Animals” projects. The findings from the projects are compared with linguistic data from Homo sapiens and with newer evidence gleaned from experiments on animal syntactic skills. The question concerning what had the so-called “Talking Animals” really done is broken down into two categories – words and (recursive) syntax. The (relative) failure of the animal projects in both categories points mainly to the fact that the core feature of language – hierarchical recursive syntax – is missing in the pseudo-linguistic feats of the animals. Keywords: language • syntax • representation • meta-representation • zoosemiotics • anthroposemiotics • talking animals • general cognition • representational systems • evolutionary discontinuity • biosemiotics © Sciendo 1. The “Talking Animals” Projects For the sake of brevity, I offer a greatly selective review of some of the more important “Talking Animals” projects. Please note that many omissions were necessary for reasons of space. The “thought climate” of the 1960s and 1970s was formed largely by the Skinnerian zeitgeist, in which it seemed possible to teach any animal to master any, or almost any, skill, including language. Perhaps riding on an ideological wave, following the surprising claims of Fossey [1] and Goodall [2] concerning primates, as well as the claims of Lilly [3] and Batteau and Markey [4] concerning dolphins, many scientists and researchers focussed on the continuities between humans and other species, while largely ignoring the discontinuities and differences. -

Do Apes and Monkeys Rely Upon Conceptual Reversibility? a Review of Studies Using Seriated Nesting Cups in Children and Nonhuman Primates
Anim Cogn (2001) 4:315–324 DOI 10.1007/s10071-001-0117-4 ORIGINAL ARTICLE Julie Johnson-Pynn · Dorothy M. Fragaszy Do apes and monkeys rely upon conceptual reversibility? A review of studies using seriated nesting cups in children and nonhuman primates Received: 2 October 2000 / Accepted after revision: 10 September 2001 / Published online: 26 October 2001 © Springer-Verlag 2001 Abstract The ability to seriate nesting cups as a sensori- Keywords Seriation · Skill development · Nonhuman motor task has posed interesting questions for cognitive primates · Perceptual-motor learning scientists. Greenfield et al. [(1972) Cognit Psychol 3:291– 310] found parallels between children’s combinatorial ac- tivity with nesting cups and patterns of phonological and Introduction grammatical constructions. The parallels suggested the possibility of a neurally based developmental homology A sizeable body of literature documents both variety and between language and instrumental action [Greenfield complexity in children’s manipulation of objects in free (1991) Behav Brain Sci 14:531–595]. Children who pre- play and in goal-oriented tasks (e.g., Bruner 1973; McCall dominantly used subassembly, a hierarchical method of 1974; Fenson et al. 1976; Wood et al. 1976). In children, combining cups, succeeded at seriating nesting cups more the ability to seriate, or order a set of objects by size from often than those who did not. Greenfield and others [e.g., smallest to largest, has been interpreted as a form of logi- Piaget and Inhelder (1969) The psychology of the child. cal cognition that stems from actions such as putting ob- Basic Books, New York; DeLoache et al. (1985) Child jects in piles, aligning objects, and arranging objects in an Dev 56:928–939] argued that success in seriation reflects array (Inhelder and Piaget 1964; Woodward 1972; Langer the child’s growing recognition of a reversible relation- 1980). -

Pdf/133/1/29/1828756/001152604772746675.Pdf by Guest on 25 September 2021
Daniel John Povinelli Behind the ape’s appearance: escaping anthropocentrism in the study of other minds Downloaded from http://direct.mit.edu/daed/article-pdf/133/1/29/1828756/001152604772746675.pdf by guest on 25 September 2021 Look at Megan. Not just at her distinc- dedicated his life to studying these tively chimpanzee features–her accen- remarkable animals–entertain the pos- tuated brow ridge, her prognathic face, sibility that their minds are, in profound her coarse black hair–but at the totality respects, radically different from our of her being: her darting eyes, her slow, own? How can I challenge the received studied movements, the gestures she wisdom of Darwin–con½rmed by my makes as her companion, Jadine, passes own initial impressions–that the mental nearby. Can there be any doubt that be- life of a chimpanzee is best compared to hind certain obvious differences in her that of a human child? appearance resides a mind nearly identi- Actually, it’s easy: I have learned to cal to our own? Indeed, is it even possi- have more respect for them than that. ble to spend an afternoon with her and I have come to see that we distort their not come to this conclusion? Upon re- true nature by conceiving of their minds flection, you will probably acknowledge as smaller, duller, less talkative versions that her mind is not identical to ours. of our own. Casting aside these insidious “But surely it’s not qualitatively differ- assumptions has been dif½cult, but it has ent, either,” you will still insist. -

Human Uniqueness in the Age of Ape Language Research1
Society and Animals 18 (2010) 397-412 brill.nl/soan Human Uniqueness in the Age of Ape Language Research1 Mary Trachsel University of Iowa [email protected] Abstract This paper summarizes the debate on human uniqueness launched by Charles Darwin’s publi- cation of The Origin of Species in 1859. In the progress of this debate, Noam Chomsky’s intro- duction of the Language-Acquisition Device (LAD) in the mid-1960s marked a turn to the machine model of mind that seeks human uniqueness in uniquely human components of neu- ral circuitry. A subsequent divergence from the machine model can be traced in the short his- tory of ape language research (ALR). In the past fifty years, the focus of ALR has shifted from the search for behavioral evidence of syntax in the minds of individual apes to participant- observation of coregulated interactions between humans and nonhuman apes. Rejecting the computational machine model of mind, the laboratory methodologies of ALR scientists Tetsuro Matsuzawa and Sue Savage-Rumbaugh represent a worldview coherent with Darwin’s continu- ity hypothesis. Keywords ape language research, artificial intelligence, Chomsky, comparative psychology, Darwin, human uniqueness, social cognition Introduction Nothing at first can appear more difficult to believe than that the more complex organs and instincts should have been perfected, not by means superior to, though analogous with, human reason, but by the accumulation of innumerable slight variations, each good for the individual possessor. (Darwin, 1989b, p. 421) With the publication of The Origin of Species (1859/1989a), Charles Darwin steered science directly into a conversation about human uniqueness previ- ously dominated by religion and philosophy. -
Participating Outlets
Participating Outlets No Name of customer Address Postal Code 1 4Fingers Terminal 3 65 Airport Boulevard, #B2-02 Changi Airport Terminal 3 819663 2 4Fingers Northpoint 930 Yishun Avenue 2, #01-15 769098 3 4Fingers Tiong Bahru Plaza 302 Tiong Bahru Road, Tiong Bahru Plaza #01-105 168732 4 4Fingers Terminal 1 80 Airport Boulevard, #03-47 Terminal 1 Departure/Transit Lounge East, Singapore Changi Airport 819642 5 4Fingers ION Orchard 2 Orchard Turn, #B4-06A 238801 6 4Fingers Jurong Point 1 Jurong West Central 2, #03-34 648886 7 4Fingers Orchard Gateway 277 Orchard Road, #01-04/05 Orchard Gateway 238858 8 4Fingers West Gate 3 Gateway Dr, #02-05 608532 9 4Fingers Plaza Singapura 68 Orchard Rd, #B1-07 238839 10 4Fingers Tampines 1 10 Tampines Central 1, #01-39/40 529536 11 4Fingers Marina Square 6 Raffles Boulevard Marian Square #02-183A 39594 12 4Fingers Causeway Point 1 Woodland Square #01-38/39 738099 13 Pepper Lunch Houganag Mall 90 Hougang Avenue 10 #B1-24/25/26 538766 14 Pepper Lunch AMK Hub 53 Ang Mo Kio Ave 3 AMK Hub #01-34 569933 15 Pepper Lunch Compass One 1 Sengkang Square, #B1-01, Compass One 545078 16 Pepper Lunch JEM 50 Jurong Gateway Road, #04-10/11/12, JEM 608549 17 Pepper Lunch Jurong Point 63 Jurong West Central 3, #B1-62/63 JP2, 648331 18 Pepper Lunch Orchard Cineileisure #05-03, 8 Grange Road 239695 19 Pepper Lunch Bedok Mall 311 New Upper Changi Road #01-05/06/07/08 467360 20 Pepper Lunch Tapines 1 10 Tampines Central 1 #B1-06 529536 21 LJS Bedok Point 799 New Upper Changi Road #01-02/03 Singapore 467351 467351 22 LJS Bugis -

MEDIA RELEASE for Immediate Release
MEDIA RELEASE For Immediate Release New National Masterplan to Encourage More to Garden - NParks takes over development of the Orchid Industry Singapore, 3 November 2017- The National Parks Board (NParks) today announced a new national gardening masterplan for Singapore which centres on promoting edible gardening. The masterplan will be implemented through both ongoing and new initiatives such as training, the expansion of the allotment gardening scheme, as well as outreach efforts like the Community Garden Festival. With a large number of community gardeners growing edibles, the Edible Horticulture Masterplan aims to increase knowledge and cultivate the community’s interest in gardening by opening up more avenues for the public to participate in Singapore’s greening efforts. During the opening of the Community Garden Festival at HortPark, NParks also unveiled HortHouse, which will serve as the new training hub for the Centre for Urban Greenery and Ecology (CUGE). This new landscape fronting HortHouse featuring eleven families of flowers and foliage will also provide an outdoor showcase of horticultural varieties to complement learning, and appeal to home gardeners. The focus on edibles taps on the immense interest in edible gardening to nurture younger gardeners. After more than a decade since the establishment of the Community in Bloom programme, community gardeners have honed their skills in growing edibles. At present, 80% of the gardening groups in HDB estates grow edibles in their community gardens. This burgeoning interest in edibles is also evident in the increasing number of entries in the NParks Community Garden Edibles Competition each year. There were a record number of 400 participants in this year’s competition, an approximately 33% increase from the previous year, in addition to an overall improvement in the quality of entries as the competition enters its third year. -

Living with Nature Content
ANNUAL REPORT 2018/2019 Living with Nature Content 02 CHAIRMAN’S MESSAGE 04 MEMBERS OF THE BOARD 06 MANAGEMENT TEAM 08 ORGANISATION STRUCTURE 10 THRIVING GREENERY 20 A BIOPHILIC HOME 28 A GROWING INTEREST 36 NURTURING THE FIELD 44 GARDEN CITY FUND 48 SUSTAINABILITY REPORT 52 FACTS & FIGURES 58 CORPORATE GOVERNANCE 60 PUBLICATIONS 66 FINANCIAL REVIEW 70 FINANCIAL STATEMENTS Forest Walk of Telok Blangah Hill Park When our History and Natural Heritage intersect n 2019, Singapore celebrates 200 years of the intersection between our history and our natural heritage – entrenched in our nature ways and nature reserves, our parks and park Iconnectors, as well as our flora and fauna. The idea for a national garden was planted in 1822 when Sir Stamford Raffles, the founder of modern Singapore, developed the first Botanical and Experimental Garden at Fort Canning. In 1859, the Singapore Botanic Gardens was established at the Tanglin site and in the 160 years past, it has flourished from a pleasure garden for the colonial community to a place cherished by all Singaporeans, a botanical institution known internationally. Singapore’s greening journey took root in the 1960s when founding Prime Minister, Mr Lee Kuan Yew planted a Mempat Tree at Farrer Circus. This kickstarted a national effort for tree planting, sowing the seed of a green home where beautiful parks and green belts would be the birthright of every Singaporean. Today, the intricate lattice of greenery we have woven into the cityscape supports thriving biodiversity and provides residents with a quality living environment. This not only draws the appreciation and marvel of visitors, but has become a part of our national identity. -
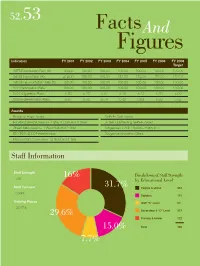
Factsand Figures
52.53 FactsAnd Figures Indicators FY 2001 FY 2002 FY 2003 FY 2004 FY 2005 FY 2006 FY 2006 Target WITS Participation Rate (%) 100.00 100.00 100.00 100.00 100.00 100.00 100.00 WITS Project Ratio (%) 212.00 188.00 190.00 181.00 172.00 171.00 170.00 WITS Implementation Ratio (%) 100.00 100.00 100.00 100.00 100.00 100.00 100.00 SSS Participation Rate 100.00 100.00 100.00 100.00 100.00 100.00 100.00 SSS Suggestion Ratio 5.30 5.23 5.07 5.10 5.20 5.20 5.00 SSS Implementation Ratio 0.67 0.63 0.78 0.60 0.62 0.59 0.53 Awards Bridge of Hope Award SHARE Gold Award Excellent Service Award – 1 Star, 6 Gold and 3 Silver 5-Year Outstanding SHARE Award Green Mark Award – 1 Gold Plus and 2 Gold Singapore HEALTH Award – Platinum ISO 9001:2000 Recertification Singapore Innovation Class National IQC Convention – 2 Gold and 1 Star Staff Information Staff Strength 16% Breakdown of Staff Strength 766 by Educational Level Staff Turnover 31.7% Degree & above 243 0.99% Diploma 115 Training Places GCE “A” Level 59 98.07% 29.6% Secondary & “O” Level 227 Primary & below 122 15.0% Total 766 7.7% Regional Parks & Recreation Areas Managed by NParks Area (ha) Area (ha) Ang Mo Kio Town Garden East 4.88 Marina Promenade 8.17 Ang Mo Kio Town Garden West 20.63 Mount Faber Park 56.46 Bedok Reservoir Park 42.62 One-North Park 3.31 Bedok Town Park 14.62 Pasir Ris Park 70.52 Bishan Park 60.24 Pasir Ris Town Park 14.01 Bukit Batok Nature Park 35.74 Pearl’s Hill City Park 8.50 Bukit Timah Nature Reserve 162.64 Pulau Ubin Recreation Area Central Catchment Nature Reserve 455.00 (including -

Pan-Homo Culture and Theological Primatology
Page 1 of 9 Original Research Locating nature and culture:Pan-Homo culture and theological primatology Author: Studies of chimpanzee and bonobo social and learning behaviours, as well as diverse 1,2 Nancy R. Howell explorations of language abilities in primates, suggest that the attribution of ‘culture’ to Affiliations: primates other than humans is appropriate. The underestimation of primate cultural and 1Saint Paul School of cognitive characteristics leads to minimising the evolutionary relationship of humans and Theology, Overland Park, other primates. Consequently my claim in this reflection is about the importance of primate Kansas, United States studies for the enhancement of Christian thought, with the specific observation that the bifurcation of nature and culture may be an unsustainable feature of any world view, which 2Department of Dogmatics and Christian Ethics, includes extraordinary status for humans (at least, some humans) as a key presupposition. University of Pretoria, Intradisciplinary and/or interdisciplinary implications: The scientific literature concerning South Africa primate studies is typically ignored by Christian theology. Reaping the benefits of dialogue Correspondence to: between science and religion, Christian thought must engage and respond to the depth of Nancy Howell primate language, social, and cultural skills in order to better interpret the relationship of nature and culture. Email: [email protected] Postal address: Introduction 4370 West 109th Street, Suite 300, Overland Park, Concentration keeps me attentive to details, but also makes me selective about what is pushed Kansas 66211-1397, to margins. Sometimes I regret what I have missed. On a visit to the Iowa Primate Learning United States Sanctuary a few years ago, I was intensely focused on committee business at hand. -

The Chimpanzee Mind: in Search of the Evolutionary Roots of the Human Mind
Anim Cogn (2009) 12 (Suppl 1):S1–S9 DOI 10.1007/s10071-009-0277-1 REVIEW The chimpanzee mind: in search of the evolutionary roots of the human mind Tetsuro Matsuzawa Received: 25 September 2008 / Revised: 3 August 2009 / Accepted: 4 August 2009 / Published online: 29 August 2009 © Springer-Verlag 2009 Abstract The year 2008 marks the 60th anniversary of Keywords Chimpanzee · Psychophysics · Comparative Japanese primatology. Kinji Imanishi (1902–1992) Wrst vis- cognitive science · Field experiment · Participation ited Koshima island in 1948 to study wild Japanese mon- observation · Ai project keys, and to explore the evolutionary origins of human society. This year is also the 30th anniversary of the Ai pro- ject: the chimpanzee Ai Wrst touched the keyboard con- Sixty years of Japanese primatology and the Ai project nected to a computer system in 1978. This paper summarizes the historical background of the Ai project, The year 2008 is the 60th anniversary of the birth of Japanese whose principal aim is to understand the evolutionary ori- primatology. Kinji Imanishi (1902–1992) Wrst arrived on gins of the human mind. The present paper also aims to Koshima island to study Japanese monkeys in the wild on 3 present a theoretical framework for the discipline called December 1948 (Matsuzawa and McGrew 2008). Imanishi comparative cognitive science (CCS). CCS is characterized and his colleagues were interested in the evolutionary origins by the collective eVorts of researchers employing a variety of human society. They thus decided to study monkey socie- of methods, together taking a holistic approach to under- ties, exploring various aspects of ecology and behavior: domi- stand the minds of nonhuman animals. -

Singapore Raptor Report February 2017
Singapore Raptor Report February 2017 Chinese Sparrowhawk, adult female, Ang Mo Kio, 17 Feb 2017, by Tan Gim Cheong. Summary for migrant species: In February, 66 individuals of 7 migrant species were recorded. While the 26 Oriental Honey Buzzards were similar to last February's numbers, the 19 Black Bazas represented a drop of more than half compared to last February. All the Black Bazas were recorded at the Punggol - Pasir Ris - Tampines area. Six Jerdon's Baza were recorded, five at Punggol on the 4th and one at Pasir Ris Park on the 12th, good numbers for this species. Of the six Peregrine Falcons recorded, two adults were photographed fighting at Seletar Airport vicinity on the 27th. Three Japanese Sparrowhawks were recorded; two of them, adult males, on the 6th at Changi Business Park and 10th at Bidadari, showed signs of moult, similar to what was observed last February, and had only 4 'fingers' instead of the usual 5 'fingers'. Five Ospreys were recorded, including three over Bukit Timah Hill on the 20th. Two Chinese Sparrowhawks were recorded, one at Kent Ridge Park on the 2nd and another, an adult female, at Ang Mo Kio on the 5th, 17th and 19th. A Crested Serpent Eagle photographed at Kent Ridge Park on the 10th by Gavan Leong turned out to be a 3rd year burmanicus, thanks to Dr Chaiyan for his expertise. This is the second occurrence of the burmanicus form, a short distance migrant from Indo-China, to Singapore. The previous record was in September and November 2014 when an individual was photographed at the Japanese Gardens. -

A Case of Imaginary Social Play by an Adolescent Male
<Article> Playing with His Leg: A Case of Imaginary Social Title Play by an Adolescent Male Chimpanzee at Bossou? Author(s) Nakamura, Michio Citation Pan Africa News (2012), 19(1): 1-3 Issue Date 2012-06 URL http://hdl.handle.net/2433/157939 Right Copyright © Pan Africa News. Type Article Textversion publisher Kyoto University Pan Africa News TThehe NNewsletterewsletter ooff tthehe CCommitteeommittee fforor tthehe CCareare aandnd CConservationonservation ooff Chimpanzees,Chimpanzees, aandnd tthehe MMahaleahale WWildlifeildlife CConservationonservation SocietySociety ISSN 1884-751X (print), 1884-7528 (online) mahale.main.jp/PAN/ JUNE 2012 VOL. 19, NO. 1 P. A. N. EDITORIAL STAFF Chief Editor: Contents Kazuhiko Hosaka, Kamakura Womenʼs University, Japan Deputy Chief Editor: <ARTICLE> Michio Nakamura, Kyoto University, Japan Playing with His Leg: A Case of Imaginary Social Play by an Adolescent Male Chimpanzee at Bossou? Associate Editors: Michio Nakamura 1 Christophe Boesch, Max-Planck Institute, Germany Jane Goodall, Jane Goodall Institute, USA <NOTE> Tetsuro Matsuzawa, Kyoto University, Japan A Wild Chimpanzee Birth at Mahale William C. McGrew, University of Cambridge, UK Koichiro Zamma, Tetsuya Sakamaki & John C. Mitani, University of Michigan, USA Rashidi Shabani Kitopeni 3 Vernon Reynolds, Budongo Forest Project, UK Yukimaru Sugiyama, Kyoto University, Japan <NOTE> Richard W. Wrangham, Harvard University, USA Ecological Aspects of Chimpanzee Insectivory in the Takeshi Furuichi, Kyoto University, Japan Budongo Forest, Uganda Sophie Hedges & William C. McGrew 6 Editorial Secretaries: Noriko Itoh, Kyoto University, Japan <NOTE> Koichiro Zamma, Great Ape Research Institute, Hayashibara, Japan Update on the Assirik Chimpanzee (Pan troglodytes Agumi Inaba, Mahale Mountains Chimpanzee Research verus) Population in Niokolo Koba National Park, Project Senegal Eiji Inoue, Kyoto University, Japan Jill D.