Life Sciences Industrial Strategy: Who’S Driving the Bus?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
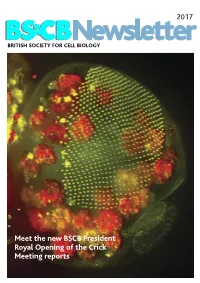
BSCB Newsletter 2017D
2017 BSCB Newsletter BRITISH SOCIETY FOR CELL BIOLOGY Meet the new BSCB President Royal Opening of the Crick Meeting reports 2017 CONTENTS BSCB Newsletter News 2 Book reviews 7 Features 8 Meeting Reports 24 Summer students 30 Society Business 33 Editorial Welcome to the 2017 BSCB newsletter. After several meeting hosted several well received events for our Front cover: years of excellent service, Kate Nobes has stepped PhD and Postdoc members, which we discuss on The head of a Drosophila pupa. The developing down and handed the reins over to me. I’ve enjoyed page 5. Our PhD and Postdoc reps are working hard compound eye (green) is putting together this years’ newsletter. It’s been great to make the event bigger and better for next year! The composed of several hundred simple units called ommatidia to hear what our members have been up to, and I social events were well attended including the now arranged in an extremely hope you will enjoy reading it. infamous annual “Pub Quiz” and disco after the regular array. The giant conference dinner. Members will be relieved to know polyploidy cells of the fat body (red), the fly equivalent of the The 2016 BSCB/DB spring meeting, organised by our we aren’t including any photos from that here. mammalian liver and adipose committee members Buzz Baum (UCL), Silke tissue, occupy a big area of the Robatzek and Steve Royle, had a particular focus on In this issue, we highlight the great work the BSCB head. Cells and Tissue Architecture, Growth & Cell Division, has been doing to engage young scientists. -

Uclpartners Academic Health Science Partnership
UCLPartners academic health science partnership Professor the Lord Ajay Kakkar, Chair, UCLPartners Professor Sir David Fish, Managing Director, UCLPartners Dr Charlie Davie, Director of UCLPartners AHSN Clare Panniker, Chief Executive, Basildon and Thurrock University Hospitals NHS Foundation Trust What is UCLPartners? Six million population 23 healthcare organisations acute and 11 higher education institutes mental health trusts; community providers and research networks 20 Clinical Commissioning Groups (CCGs) Industry partnerships in research and 26 boroughs and local councils translation of innovation into health and wealth 2 Local Enterprise Partnership – key challenge • Working with the London Enterprise Panel, established by the Mayor of London • Professor Stephen Caddick, Vice Provost (Enterprise), UCL, is the only academic representative on the Panel • Key challenges of the panel: to compete with Boston and San Francisco; improve access to the NHS market to increase venture capital • How UCLPartners is contributing: working with industry to co-create technology and devices; creating long-term partnerships with industry and giving confidence to entrepreneurs, e.g. through new business models and procurement initiatives • Other areas of joint working: MedCity, Care City, London Health Commission, three London AHSNs and preparing to enable the success of the Francis Crick Institute 3 Defragmenting the pathway – an integrated journey to transform healthcare through innovation into practice Bringing together formal designations under -

Applying Polygenic Risk Scoring for Psychiatric Disorders to a Large
http://www.diva-portal.org This is the published version of a paper published in . Citation for the original published paper (version of record): de Jong, S., Abdalla Diniz, M J., Saloma, A., Gadelha, A., Santoro, M L. et al. (2018) Applying polygenic risk scoring for psychiatric disorders to a large family with bipolar disorder and major depressive disorder Communications Biology, 1: 163 https://doi.org/10.1038/s42003-018-0155-y Access to the published version may require subscription. N.B. When citing this work, cite the original published paper. Permanent link to this version: http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-157800 ARTICLE DOI: 10.1038/s42003-018-0155-y OPEN Applying polygenic risk scoring for psychiatric disorders to a large family with bipolar disorder and major depressive disorder Simone de Jong1,2, Mateus Jose Abdalla Diniz3,4, Andiara Saloma3,4, Ary Gadelha3, Marcos L. Santoro5, 1234567890():,; Vanessa K. Ota3,5, Cristiano Noto3, Major Depressive Disorder and Bipolar Disorder Working Groups of the Psychiatric Genomics Consortium#, Charles Curtis1,2, Stephen J. Newhouse2,6,7, Hamel Patel2,6, Lynsey S. Hall8, Paul F. O`Reilly1, Sintia I. Belangero3,5, Rodrigo A. Bressan3 & Gerome Breen 1,2 Psychiatric disorders are thought to have a complex genetic pathology consisting of interplay of common and rare variation. Traditionally, pedigrees are used to shed light on the latter only, while here we discuss the application of polygenic risk scores to also highlight patterns of common genetic risk. We analyze polygenic risk scores for psychiatric disorders in a large pedigree (n ~ 260) in which 30% of family members suffer from major depressive disorder or bipolar disorder. -

Clinical Academic Leadership in COVID-19: a Rapid Leader: First Published As 10.1136/Leader-2020-000292 on 1 July 2020
Brief report Clinical academic leadership in COVID-19: a rapid leader: first published as 10.1136/leader-2020-000292 on 1 July 2020. Downloaded from response to sharing emerging insights in intensive care Nirandeep Rehill,1,2 Amanda Begley,1 Katie Mantell,1 C Michael Roberts1 1UCL Partners, London, UK ABSTRACT assume that role. UCLPartners is a partnership of 2 NIHR Applied Health Research Background The COVID-19 pandemic has raised a 23 National Health Service (NHS) trusts and 9 North Thames, London, UK wide range of challenges for health systems around the higher education institutions covering north central world and the National Health Service in England has and east London and parts of Essex, Hertfordshire Correspondence to Dr Amanda Begley, UCL been no exception. A significant proportion of infected and Bedfordshire. Governed by a partnership board Partners, London W1T 7HA, UK; cases require intensive care unit support and have a high and led by clinical academics supported by a corpo- amanda. begley@ uclpartners. mortality rate. In the early stages of the pandemic, there rate senior team, its purpose is to bring together com was neither an evidence base nor a clinical consensus on organisations to work collaboratively for the health and care of the population served. Received 18 May 2020 the optimal management of patients in this setting. Revised 9 June 2020 Interventions Responding to requests for assistance Accepted 11 June 2020 to address this evidence gap, UCLPartners, an Academic SHARING EMERGING INSIGHTS Health Science Partnership, working in collaboration with Through our partnership, we received intelligence other organisations including National Institute of Health that clinicians were concerned that the traditional Research Applied Research Collaboration North Thames, management of ARDS and other complications was developed a clinical academic team to synthesise clinical not having expected outcomes against this novel learning in real time. -

Dr Charlie Davie, Managing Director, Uclpartners
Collaborating with industry and researchers to deliver improved healthcare Dr Charlie Davie, Managing Director, UCLPartners UCLPartners – a partnership organisation Parts of London, Bedfordshire, Hertfordshire and Essex 6 million 46 11 population Healthcare Higher education organisations and partners clinical commissioners 844 100s 6 primary care practices industry partnerships Sustainability and Transformation Partnerships A unique business model UCLPartners aligns the following functions in one partnership: • Academic Health Science Centre (AHSC) • Academic Health Science Network (AHSN) • NIHR Collaboration for Applied Health Research and Care (CLAHRC/ARC) • Commercial Trials Prime Site • NIHR Clinical Research Network (CRN) • Genomic Medicine Centre 5 Life Sciences Industrial Strategy • Science: Continued support for the science base, maintaining strength and international competitiveness. • Growth: An environment that encourages companies to start and grow, building on strengths across the UK, including expansion of manufacturing in the sector. • NHS: NHS and industry collaboration, facilitating better care for patients through better adoption of innovative treatments and technologies. • Data: Making the best use of data and digital tools to support research and better patient care. • Skills: Ensuring that the sector has access to a pool of talented people to support its aims through a strong skills strategy. 6 7 The Accelerated Access Review Aim: To speed up access to innovation and grow the UK life sciences industry Recommendations in five key areas: 1. Patients, clinicians and charities to be the key drivers in innovation 2. A new accelerated access pathway will prioritise innovations 3. Open and transparent pathways to bring forward medtech, digital and diagnostics 4. Driving innovation through NHS planning, increasing capacity, clinical leadership and incentives 5. -

Honorary Degrees 2011
Honorary Degrees 2011 The full list of those who received honorary degrees is as follows: COMMEMORATION DAY, WEDNESDAY 15 JUNE 2011 DEGREE OF DOCTOR OF DIVINITY (DD) Very Reverend David LUNAN, Clerk to the Presbytery of Glasgow DEGREE OF DOCTOR OF LAWS (LLD) Baroness Brenda HALE, Barrister and Judge DEGREE OF DOCTOR OF LETTERS (DLitt) Professor Toshiyuki TAKAMIYA, Emeritus Professor, Keio University, Tokyo DEGREE OF DOCTOR OF SCIENCE (DSc) Professor Sir Leszek BORYSIEWICZ, Vice-Chancellor of the University of Cambridge & former Chief Executive of the MRC Professor Victor J DZAU, James B Duke Professor of Medicine, Duke University, USA DEGREE OF DOCTOR OF THE UNIVERSITY (DUniv) Lord John MCFALL of Alcuith, Former MP for West Dunbartonshire SUMMER GRADUATIONS DEGREE OF DOCTOR OF LETTERS (DLitt) 5 March FAN ZENG, Prominent Artist in traditional Chinese Art DEGREE OF DOCTOR OF LETTERS (DLitt) GSA Graduation 17 June Katrina BROWN, Director of the Glasgow International Festival of Visual Arts DEGREE OF DOCTOR OF LETTERS (DLitt) 23 June 11am Professor Simon BLACKBURN, Faculty of Philosophy, University of Cambridge DEGREE OF DOCTOR OF LETTERS (DLitt) 28 June 11am Professor Sir Hilary BECKLES, University of West Indies, West Indies Professor Caroline Walker BYNUM, Emeritus Professor, Columbia University, USA DEGREE OF DOCTOR OF LETTERS (DLitt) 28 June 4pm Armando IANNUCCI, Comedy Writer, Producer and Director DEGREE OF DOCTOR OF LETTERS (DLitt) 6 July Crichton Campus Alastair REID, Writer and Translator DEGREE OF DOCTOR OF SCIENCE (DSc) 10 February -

Health Tech Innovator Drdoctor Gains Three New NHS Contracts
Press release Health tech innovator DrDoctor gains three new NHS contracts Patient appointment innovator DrDoctor is working with three new major NHS Trusts: • A major children’s hospital, where the system is being configured in record time and will manage the Trust’s 150000 specialist outpatient appointments • Whittington Health NHS Trust, covering all appointments across the Trust • University Hospital Southampton NHS Foundation Trust, DrDoctor is piloting in Paediatrics to demonstrate an estimated 30% DNA rate reduction DrDoctor supports hospitals with digital transformation. Its system allows patients to view, change and schedule outpatient appointments themselves, online, on smartphone or by conversational SMS. DrDoctor chief executive Tom Whicher said: “We are thrilled to support three more forward thinking Trusts in their journey to provide effective, digital first, patient centric services. All three implementations will deliver, in year cash savings, increase patient satisfaction and form a strong foundation for future digital transformation.” DrDoctor reduces the number of missed and unscheduled appointments and allows patients to opt in to receiving paperless communications, releasing print and postage savings to Trusts. New analysis at Guy’s and St Thomas’ NHS Foundation Trust has revealed that in one year the Trust has saved £2.2m, and patient satisfaction is 96 percent. DrDoctor is supporting a major London children’s hospital’s strategic plan to become the leading paediatric centre in the world. DrDoctor was engaged in January 2017 to deploy its Appointment Management Platform. DrDoctor’s platform is a fundamental shift away from an inconsistent approach to appointment management. The platform will go-live in the summer of 2017. -

'Astrazeneca' Covid-19 Vaccine
Medicines Law & Policy How the ‘Oxford’ Covid-19 vaccine became the ‘AstraZeneca’ Covid-19 vaccine By Christopher Garrison 1. Introduction. The ‘Oxford / AstraZeneca’ vaccine is one of the world’s leading hopes in the race to end the Covid-19 pandemic. Its history is not as clear, though, as it may first seem. The media reporting about the vaccine tends to focus either on the very small (non-profit, academic) Jenner Institute at Oxford University, where the vaccine was first invented, or the very large (‘Big Pharma’ firm) AstraZeneca, which is now responsible for organising its (non-profit) world-wide development, manufacture and distribution. However, examining the intellectual property (IP) path of the vaccine from invention to manufacture and distribution reveals a more complex picture that involves other important actors (with for-profit perspectives). Mindful of the very large sums of public money being used to support Covid-19 vaccine development, section 2 of this note will therefore contextualise the respective roles of the Jenner Institute, AstraZeneca and these other actors, so that their share of risk and (potential) reward in the project can be better understood. Section 3 provides comments as well as raising some important questions about what might yet be done better and what lessons can be learned for the future. 2. History of the ‘Oxford / AstraZeneca’ vaccine. 2.1 Oxford University and Oxford University Innovation Ltd. The Bayh-Dole Act (1980) was hugely influential in the United States and elsewhere in encouraging universities to commercially exploit the IP they were generating by setting up ‘technology transfer’ offices. -

Oxford Medicine
Oxford Medicine THE NEWSLETTER OF THE OXFORD MEDICAL ALUMNI OXFORD MEDICINE • DECEMBER 2019 Courtesy of Ludwig Cancer Research of Ludwig Cancer Courtesy The Regius Professor Sir Tingewick is Does reflects on Peter Ratcliffe 80! Developmental 45 years in FRS, Nobel Dyslexia Really medicine Laureate Exist? 2 / OXFORD MEDICINE DECEMBER 2019 President’s Piece Welcome to the December Sir William Osler’s Centenary commemorations will issue of Oxford Medicine, the continue throughout the year in Oxford and beyond. newsletter for Oxford Medical The Osler Club is the first of a number of thriving Alumni (OMA) who have postgraduate Oxford medical societies we plan to feature. trained, taught, or worked at Professor Terence Ryan summarises this year’s five Osler Oxford. Professor John Morris, Club seminars exploring the Oslerian theme ‘For Health OMA president for the past and Wellbeing, Science and Humanities are one’. six years, handed the baton Tingewick is 80 this year. In 2019, as in 1939, Tingewick to me in September. It is a Dr Lyn Williamson, is still the most inclusive Oxford clinical student society. OMA President daunting task to take over from This year, every first year clinical student took part - someone so beloved and so that is 165! The show was a triumph of teamwork and respected, who has taught anatomy to generations talent. The legacy of camaraderie will last a lifetime - as of Oxford students and postgraduates, and shaped witnessed by the 80th anniversary celebrations. Dr Derek the preclinical school for many years. With his Roskell, Senior Tingewick Member for 25 years adds his characteristic kindness and wisdom, he said: ‘You will be fine - and I will be there to advise you’. -

Developing the Evidence Base for London's Local Industrial Strategy
Developing the evidence base for London’s Local Industrial Strategy - Interim report August 2019 Developing the evidence base for London’s Local Industrial Strategy - Interim report copyright Greater London Authority August 2019 Published by Greater London Authority City Hall The Queens Walk London SE1 2AA www.london.gov.uk Tel 020 7983 4000 Minicom 020 7983 4000 ISBN 978-1-84781-719-8 Cover photograph © Shutterstock For more information about this publication, please contact: GLA Economics Tel 020 7983 4000 Email [email protected] GLA Economics provides expert advice and analysis on London’s economy and the economic issues facing the capital. Data and analysis from GLA Economics form a basis for the policy and investment decisions facing the Mayor of London and the GLA group. GLA Economics uses a wide range of information and data sourced from third party suppliers within its analysis and reports. GLA Economics cannot be held responsible for the accuracy or timeliness of this information and data. The GLA will not be liable for any losses suffered or liabilities incurred by a party as a result of that party relying in any way on the information contained in this report. Acknowledgements GLA Economics would like to thank all colleagues at the GLA and in other organisations who contributed to this report, including: • Members of the Analytical Working Group, especially the ONS regional statisticians (Sukriti Verma and Tom Liu) for all their work on developing London-relevant statistics; • Gerard Burgess and Jorn Peters in the GLA Planning Directorate for their very valuable comments and contributions to Chapters 7 and 8; • Professor Stephen Roper at the Enterprise Research Centre (Warwick Business School) for his valuable advice on Chapter 6; • The LIS Evidence Base Independent Expert Panel – Professor Riccardo Crescenzi, Dr. -

Presidential Perspectives
Presidential perspectives Professor Sir John Bell talks to Geoff Watts about medical science – from the recent past to the near future As part of the Academy of Medical Sciences’ 10th anniversary celebrations, we asked Dr Geoff Watts FMedSci to conduct a series of interviews with the President, Professor Sir John Bell FRS PMedSci. The broad theme was‘medical science, from the recent past to the near future’. Captured here are some highlights from the interviews, giving Sir John’s perspectives on molecular medicine, stem cells and diseases of the developed and developing world, as well as the role of the Academy. Molecular medicine principal research interests lie within one of the topics set to change the way medicine operates: ‘The rapid advances in human molecular the molecular basis of disease. So a good place genetics seen over the past five years indicate to begin this skim through some of his thinking. that within the next decade genetic testing will be used widely for predictive testing in healthy ‘I think there’s a rule that big people and for diagnosis and management of discoveries in biomedicine take at patients.’ – John Bell. BMJ (1998) 316, 618. least 20 years to impact in the clinic.‘ Making predictions on the record is risky - but Professor Sir John Bell has the self-assurance to That he sees the sequencing of the human offer an occasional hostage to fortune. As the genome as a scientific milestone comes as no date on the quote above from the BMJ reveals, surprise. But a clinical one as well?‘I think there’s not every prediction hits all the bull’s-eyes dead a rule that big discoveries in biomedicine take centre. -

Gagna A. & Ch. Van Heck
GAGNA A. & CH. VAN HECK PRIZE 2015 18 NOVEMBER 2015 Contact: Mr Bruno MORAUX F.R.S.-FNRS [email protected] 02/504.92.40 GAGNA A. & CH. VAN HECK PRIZE The triennial and international « Gagna A. & Ch. Van Heck Prize », amounting to 75.000 €, rewards a scientist or a Medical Doctor, in recognition of a research work which has contributed to the treatment of a disease currently incurable, or which has raised hopes for curing the disease. This Prize was first granted in 2003 and constitutes one of the most prestigious Belgian Prize in biomedical science. The Prize is awarded to: Stephen P. JACKSON Ph.D. in Molecular Biology, University of Edinburgh (UK) Head of Cancer Research UK Laboratories, The Gurdon Institute, University of Cambridge (UK) Professor of Biology, University of Cambridge (UK) Identification of key mechanisms for the detection, signaling and repair of DNA damages. Professor Stephen Jackson’s pioneering academic research has provided us with many of the key principles by which cells detect DNA damage, signal its presence and mediate its repair. His discoveries have had particularly major impacts on our understanding of how human cells deal with the most toxic of all DNA damages: the DNA double-strand break (DSB). In addition to establishing some of the most important foundations of present-day DNA repair research, Jackson’s work has also shown us how cellular dysfunction and disease arise when DNA repair or DNA-damage signaling goes awry. Additionally, his work has led to the development of an innovative new approach to cancer therapy that kills cancer cells whose ability to repair DNA damage has been compromised.