Glossary and Tutorial of Xenobiotic Metabolism Terms Used During
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparison of the Effects on Mrna and Mirna Stability Arian Aryani and Bernd Denecke*
Aryani and Denecke BMC Research Notes (2015) 8:164 DOI 10.1186/s13104-015-1114-z RESEARCH ARTICLE Open Access In vitro application of ribonucleases: comparison of the effects on mRNA and miRNA stability Arian Aryani and Bernd Denecke* Abstract Background: MicroRNA has become important in a wide range of research interests. Due to the increasing number of known microRNAs, these molecules are likely to be increasingly seen as a new class of biomarkers. This is driven by the fact that microRNAs are relatively stable when circulating in the plasma. Despite extensive analysis of mechanisms involved in microRNA processing, relatively little is known about the in vitro decay of microRNAs under defined conditions or about the relative stabilities of mRNAs and microRNAs. Methods: In this in vitro study, equal amounts of total RNA of identical RNA pools were treated with different ribonucleases under defined conditions. Degradation of total RNA was assessed using microfluidic analysis mainly based on ribosomal RNA. To evaluate the influence of the specific RNases on the different classes of RNA (ribosomal RNA, mRNA, miRNA) ribosomal RNA as well as a pattern of specific mRNAs and miRNAs was quantified using RT-qPCR assays. By comparison to the untreated control sample the ribonuclease-specific degradation grade depending on the RNA class was determined. Results: In the present in vitro study we have investigated the stabilities of mRNA and microRNA with respect to the influence of ribonucleases used in laboratory practice. Total RNA was treated with specific ribonucleases and the decay of different kinds of RNA was analysed by RT-qPCR and miniaturized gel electrophoresis. -

Enzymes Handling/Processing
Enzymes Handling/Processing 1 Identification of Petitioned Substance 2 3 This Technical Report addresses enzymes used in used in food processing (handling), which are 4 traditionally derived from various biological sources that include microorganisms (i.e., fungi and 5 bacteria), plants, and animals. Approximately 19 enzyme types are used in organic food processing, from 6 at least 72 different sources (e.g., strains of bacteria) (ETA, 2004). In this Technical Report, information is 7 provided about animal, microbial, and plant-derived enzymes generally, and more detailed information 8 is presented for at least one model enzyme in each group. 9 10 Enzymes Derived from Animal Sources: 11 Commonly used animal-derived enzymes include animal lipase, bovine liver catalase, egg white 12 lysozyme, pancreatin, pepsin, rennet, and trypsin. The model enzyme is rennet. Additional details are 13 also provided for egg white lysozyme. 14 15 Chemical Name: Trade Name: 16 Rennet (animal-derived) Rennet 17 18 Other Names: CAS Number: 19 Bovine rennet 9001-98-3 20 Rennin 25 21 Chymosin 26 Other Codes: 22 Prorennin 27 Enzyme Commission number: 3.4.23.4 23 Rennase 28 24 29 30 31 Chemical Name: CAS Number: 32 Peptidoglycan N-acetylmuramoylhydrolase 9001-63-2 33 34 Other Name: Other Codes: 35 Muramidase Enzyme Commission number: 3.2.1.17 36 37 Trade Name: 38 Egg white lysozyme 39 40 Enzymes Derived from Plant Sources: 41 Commonly used plant-derived enzymes include bromelain, papain, chinitase, plant-derived phytases, and 42 ficin. The model enzyme is bromelain. -

Enzyme2 File
Enzymes Theories of enzyme substrate interaction 2 Theories of enzyme substrate interaction . There are two proposed methods by which enzymes bind to their substrate molecules: 1- Lock & key model 2-Induced fit model 3 Theories of enzyme substrate interaction 1-lock & key model (Template hypothesis for enzyme action): The substrate fits to binding site of enzyme as a key fits into proper lock . Enzyme & substrate possess specific complementary geometric & rigid shapes fit exactly into one another. This explains enzyme specificity, but fails to explain the stabilization of the transition state & allosteric regulation & inhibition. 4 1-lock & key model : 5 Theories of enzyme substrate interaction 2-Induce fit model More flexible model than lock & key model. Enzymes are flexible structures The active site modified as the [S] interacts with the anzyme (active site of enzyme become complementary to S) Model explains enzyme specificty & stabilization of transition state, regulation & inhibition. 6 2-Induce fit model 7 Enzyme Inhibition 8 Enzyme inhibitors: Any substance that can diminish the velocity of an enzyme-catalyzed reaction. 9 Types of inhibition Irreversible Reversible inhibitors inhibitors 10 Types of inhibition 1-Irreversible inhibitors : Bind to enzymes through covalent bond (inhibited enzyme does not regain activity upon dilution of enzyme-inhibitor complex) 11 Type of inhibition 2-Reversible inhibitors: Bind to enzymes through noncovalent bonds, thus dilution of the enzyme–inhibitor complex results in dissociation of the reversibly bound inhibitor, and recovery of enzyme activity. 12 Reversible inhibitors 1. Competitive inhibition 2. Non competitive inhibition 3. Uncompeptitive inhibition 13 1-Competitive inhibition The inhibitor and substrate compete for the same active site on the enzyme as a result of similarity in structure. -
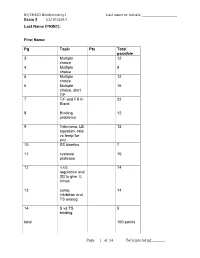
BI/CH421 Biochemistry I Last Name Or Initials: ______Exam 3 11/10/2014 Last Name (PRINT)
BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in 22 Blank 8 Binding 12 problems 9 Yakimima, LB 12 equation, rate vs temp for enz 10 SS kinetics 7 11 cysteine 15 protease 12 ∆∆G, 14 regulation and [S] to give ¾ Vmax 13 comp. 14 inhibition and TS analog 14 S vs TS 5 binding total 150 points Page of 11 4 Total pts for pg _________ BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Instructions: READ INSTRUCTIONS BEFORE BEGINNING EXAM. 1) Carefully read question before answering. Often I highlight very important information so please make note when I do so to make sure you are answering the question correctly. 2) Write your FULL name above and at least your last name or initials on every page. 3) Write all of your answers on the exam paper itself in the space provided. If you need additional space, you can write on the back of the SAME page. If you do this, you must write “ON BACK” so that we know where to look for your answer. 4) Your answers should be brief and legible. A correct answer that cannot be read cannot receive full credit. Additionally, extremely lengthy responses containing both correct and incorrect statements will be graded accordingly. Meaning, if you answer the question correctly but if you go on to write a “kitchen sink” response containing incorrect information, you will not receive full credit for that answer. -

The Serpintine Solution
& Experim l e ca n i t in a l l C C f a Journal of Clinical & Experimental o r d l i a o n l o r g u Lucas et al., J Clin Exp Cardiolog 2017, 8:1 y o J Cardiology ISSN: 2155-9880 DOI: 10.4172/2155-9880.1000e150 Editorial Open Access The Serpintine Solution Alexandra Lucas, MD, FRCP(C)1,2,*, Sriram Ambadapadi, PhD1, Brian Mahon, PhD3, Kasinath Viswanathan, PhD4, Hao Chen, MD, PhD5, Liying Liu, MD6, Erbin Dai, MD6, Ganesh Munuswami-Ramanujam, PhD7, Jacek M. Kwiecien, DVM, MSc, PhD8, Jordan R Yaron, PhD1, Purushottam Shivaji Narute, BVSc & AH, MVSc, PhD1,9, Robert McKenna, PhD10, Shahar Keinan, PhD11, Westley Reeves, MD, PhD12, Mark Brantly, MD, PhD13, Carl Pepine, MD, FACC14 and Grant McFadden, PhD1 1Center for Personalized Diagnostics, Biodesign Institute, Arizona State University, Tempe AZ, USA 2Saint Josephs Hospital, Dignity Health Phoenix, Phoenix, AZ, USA 3NIH/ NIDDK, Bethesda MD, USA 4Zydus Research Centre, Ahmedabad, India 5Department of tumor surgery, The Second Hospital of Lanzhou University, Lanzhou, Gansu, P.R.China 6Beth Israel Deaconess Medical Center, Harvard, Boston, MA, USA 7Interdisciplinary Institute of Indian system of Medicine (IIISM), SRM University, Chennai, Tamil Nadu, India 8MacMaster University, Hamilton, ON, Canada 9Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda MD, USA 10Department of Molecular Genetics and Microbiology, University of Florida, Gainesville, FL, USA 11Cloud Pharmaceuticals, Durham, North Carolina, USA 12Division of Rheumatology, University of Florida, -

The Coffee Protective Effect on Catalase System in the Preneoplastic Induced Rat Liver
Hindawi Publishing Corporation Journal of Chemistry Volume 2016, Article ID 8570321, 9 pages http://dx.doi.org/10.1155/2016/8570321 Research Article The Coffee Protective Effect on Catalase System in the Preneoplastic Induced Rat Liver Cristiana Schmidt de Magalhães,1 Jéssica Emi Takarada,1 Nathália Costa Carvalho,1 Dayene do C. Carvalho,2 Felipe Lopes de Andrade,1 Eric Batista Ferreira,1 Pedro Orival Luccas,2 and Luciana Azevedo3 1 Exact Sciences Institute, Federal University of Alfenas, Rua Gabriel Monteiro da Silva 700, Centro, 37130-000 Alfenas, MG, Brazil 2Chemistry Institute, Federal University of Alfenas, Rua Gabriel Monteiro da Silva 700, Centro, 37130-000 Alfenas, MG, Brazil 3Nutrition Faculty, Federal University of Alfenas, Rua Gabriel Monteiro da Silva 700, Centro, 37130-000 Alfenas, MG, Brazil Correspondence should be addressed to Cristiana Schmidt de Magalhaes;˜ [email protected] Received 15 October 2015; Revised 2 March 2016; Accepted 28 March 2016 Academic Editor: Philippe Jeandet Copyright © 2016 Cristiana Schmidt de Magalhaes˜ et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. This study aimed to evaluate the effect of organic/conventional coffee in liver tissues in the cancer process, taking into account the level and activities of catalase. The experiments were carried out with 8 groups of rats during 12 weeks. They received two injections of ethylenediaminetetraacetic acid solution 1.5% (v/v) prepared in 0.9% NaCl or 1,2-dimethylhydrazine (DMH) subcutaneous dose −1 −1 of 40 mg⋅kg ⋅bw for 2 weeks. -

Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice
pharmaceutics Review Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice Malavika Deodhar 1, Sweilem B Al Rihani 1 , Meghan J. Arwood 1, Lucy Darakjian 1, Pamela Dow 1 , Jacques Turgeon 1,2 and Veronique Michaud 1,2,* 1 Tabula Rasa HealthCare Precision Pharmacotherapy Research and Development Institute, Orlando, FL 32827, USA; [email protected] (M.D.); [email protected] (S.B.A.R.); [email protected] (M.J.A.); [email protected] (L.D.); [email protected] (P.D.); [email protected] (J.T.) 2 Faculty of Pharmacy, Université de Montréal, Montreal, QC H3C 3J7, Canada * Correspondence: [email protected]; Tel.: +1-856-938-8697 Received: 5 August 2020; Accepted: 31 August 2020; Published: 4 September 2020 Abstract: In an ageing society, polypharmacy has become a major public health and economic issue. Overuse of medications, especially in patients with chronic diseases, carries major health risks. One common consequence of polypharmacy is the increased emergence of adverse drug events, mainly from drug–drug interactions. The majority of currently available drugs are metabolized by CYP450 enzymes. Interactions due to shared CYP450-mediated metabolic pathways for two or more drugs are frequent, especially through reversible or irreversible CYP450 inhibition. The magnitude of these interactions depends on several factors, including varying affinity and concentration of substrates, time delay between the administration of the drugs, and mechanisms of CYP450 inhibition. Various types of CYP450 inhibition (competitive, non-competitive, mechanism-based) have been observed clinically, and interactions of these types require a distinct clinical management strategy. This review focuses on mechanism-based inhibition, which occurs when a substrate forms a reactive intermediate, creating a stable enzyme–intermediate complex that irreversibly reduces enzyme activity. -

„Transformation Von Phospholipiden Durch Phospholipasen A1 Und
„Transformation von Phospholipiden durch Phospholipasen A1 und Phospholipasen D“ Kumulative Dissertation zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) vorgelegt der Naturwissenschaftlichen Fakultät I Biowissenschaften der Martin-Luther-Universität Halle-Wittenberg von Martin Dippe geboren am 3. August 1981 in Wernigerode (Harz) Gutachter: 1. Prof. Renate Ulbrich-Hofmann (Martin-Luther-Universität Halle-Wittenberg) 2. Prof. Ingo Heilmann (Martin-Luther-Universität Halle-Wittenberg) 3. Prof. Uwe T. Bornscheuer (Ernst-Moritz-Arndt-Universität Greifswald) Halle (Saale), den 08. Dezember 2011 Inhaltsverzeichnis Inhaltsverzeichnis Inhaltsverzeichnis .............................................................................................................. 1 Abkürzungsverzeichnis ..................................................................................................... 2 1 Einleitung und Zielstellung .......................................................................................... 3 2 Theoretischer Teil ....................................................................................................... 5 2.1. Phospholipide – Molekularer Bau, Verwendung und chemische Synthese .... 6 2.2. Phospholipase-vermittelte Modifizierung von Phospholipiden ....................... 8 2.3. PLD-Enzyme............................................................................................... 10 2.3.1. Funktion und Mechanismus ............................................................ 10 2.3.2. Kopfgruppenaustausch -

Morelloflavone and Its Semisynthetic Derivatives As Potential Novel Inhibitors of Cysteine and Serine Proteases
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/276087209 Morelloflavone and its semisynthetic derivatives as potential novel inhibitors of cysteine and serine proteases ARTICLE in JOURNAL OF MEDICINAL PLANT RESEARCH · APRIL 2015 Impact Factor: 0.88 · DOI: 10.5897/JMPR2014.5641 DOWNLOADS VIEWS 2 17 8 AUTHORS, INCLUDING: Ihosvany Camps Claudio Viegas-jr Universidade Federal de Alfenas Universidade Federal de Alfenas 35 PUBLICATIONS 128 CITATIONS 6 PUBLICATIONS 22 CITATIONS SEE PROFILE SEE PROFILE Available from: Ihosvany Camps Retrieved on: 08 September 2015 Vol. 9(13), pp. 426-434, 3 April, 2015 DOI: 10.5897/JMPR2014.5641 Article Number: A42115152263 ISSN 1996-0875 Journal of Medicinal Plants Research Copyright © 2015 Author(s) retain the copyright of this article http://www.academicjournals.org/JMPR Full Length Research Paper Morelloflavone and its semisynthetic derivatives as potential novel inhibitors of cysteine and serine proteases Vanessa Silva Gontijo1, Jaqueline Pereira Januário1, Wagner Alves de Souza Júdice2, Alyne Alexandrino Antunes2, Ingridy Ribeiro Cabral1, Diego Magno Assis3, Maria Aparecida Juliano3, Ihosvany Camps4, Marcos José Marques4, Claudio Viegas Junior1 and Marcelo Henrique dos Santos1* 1Department of Exact Science, Laboratory of Phytochemistry and Medicinal Chemistry, Federal University of Alfenas, MG, Brazil. 2Interdisciplinary Center of Biochemical Investigation, Mogi das Cruzes University, Mogi das Cruzes, SP, Brazil. 3Department of Biophysics, Federal University of São Paulo, SP, Brazil. 4Department of Biological Sciences, Laboratory of Molecular Biology, Federal University of Alfenas, MG, Brazil. Received 9 October, 2014; Accepted 11 March, 2015 This article reports the three biflavonoids isolated from the fruit pericarp of Garcinia brasiliensis Mart. -

Study Protocol
Full title: A Sequential Phase I study of MEK1/2 inhibitors PD-0325901 or Binimetinib combined with cMET inhibitor PF-02341066 in Patients with RAS Mutant and RAS Wild Type (with aberrant c-MET) Colorectal Cancer Short title: MErCuRIC1: MEK and MET Inhibition in Colorectal Cancer Protocol Version & date: Version 9.0; 29Oct2018 Sponsor Protocol Number: OCTO_049 Ethics Number: 14/SC/1010 EudraCT Number: 2014-000463-40 Funding Reference Number 602901-2 Sponsored by the University of Oxford Funder: European Commission’s Seventh Framework Program (FP7) Confidentiality Statement This document contains confidential information that must not be disclosed to anyone other than the Sponsor, the Trials Office, the Investigator Team, host NHS Trust(s), regulatory authorities, and members of the Research Ethics Committee. MErCuRIC1_Protocol_V9.0_29Oct2018 Protocol Template V3.0_18Feb2013 Page 1 of 121 MErCuRIC1 Confidential GENERAL CONTACT INFORMATION Trial Office (OCTO) MErCuRIC Trial Office Oncology Clinical Trials Office (OCTO) Department of Oncology, The University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford Tel: +44 (0)1865 227194 Email: [email protected] Website: http://www.oncology.ox.ac.uk/research/oncology- clinical-trials-office-octo Chief Investigator Professor Mark Middleton Department of Oncology, University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford OX3 7LE Tel: +44 (0)1865 235 315 Email : [email protected] Investigator Professor Richard Wilson and -

Immobilization of Trypsin for Peptide Synthesis and Hydrolysis Reactions
IMMOBILIZATION OF TRYPSIN FOR PEPTIDE SYNTHESIS AND HYDROLYSIS REACTIONS zur Erlangung des akademischen Grades eines DOKTORS DER INGENIEURWISSENSCHAFTEN (Dr.-Ing.) der Fakultät für Chemieingenieurwesen und Verfahrenstechnik des Karlsruher Instituts für Technologie (KIT) vorgelegte genehmigte DISSERTATION von Dipl. Biol. (t.o.) Julia Andre geb. Stolarow aus Odessa, Ukraine Referent: Prof. Dr. rer. nat. Christoph Syldatk Korreferent: Prof. Dr.-Ing. Rudolf Hausmann Tag der mündlichen Prüfung: 19. November 2015 Maybe I will be something that you would be good at Für meine Eltern Acknowledgements I wish to express my sincere thanks to people who continuously supported and guided me throughout my work. Prof. Dr. rer. nat. Christoph Syldatk for giving me the opportunity to conduct my research work at his group, his guidance and his enormous scientific knowledge giving me a great opportunity to learn. Prof. Dr.-Ing. Rudolf Hausmann for his supervision, productive scientific discussions, his motivating personality and professional advice helping me to overcome many obstacles in my research work. Former and current members of the Technical Biology group for the great friendly and supporting atmosphere during my work and spare time: Dr.-Ing. Ines Schulze, Dr. rer. nat. Mareike Perzborn, Laura Krämer, Dr.-Ing. Berna Gerçe, Dr.-Ing. Martin Pöhnlein, Dipl.-Ing. Melanie Gerlitzki, Dipl.-Biotechnol. Johannes Kügler, Dr.-Ing. Marius Henkel, Dr. rer. nat. Markus Andre, Dr.-Ing. Ulrike Engel, M. Sc. Janina Beuker, Dipl.-Ing. Michaela Zwick, M. Sc. Judit Willenbacher, M. Sc. Sarah Dold, M. Sc. Christin Slomka, Sandra Baumann, Dr.-Ing. Katrin Ochsenreither, Dipl.-Ing. Florian Oswald, Desiree Westermann, Werner Mandel, Harald Gotzmann, Siegfried Almstedt, Katja Rupp, Susanne Warth, M. -

Potent Inhibition of Monoamine Oxidase B by a Piloquinone from Marine-Derived Streptomyces Sp. CNQ-027
J. Microbiol. Biotechnol. (2017), 27(4), 785–790 https://doi.org/10.4014/jmb.1612.12025 Research Article Review jmb Potent Inhibition of Monoamine Oxidase B by a Piloquinone from Marine-Derived Streptomyces sp. CNQ-027 Hyun Woo Lee1, Hansol Choi2, Sang-Jip Nam2, William Fenical3, and Hoon Kim1* 1Department of Pharmacy and Research Institute of Life Pharmaceutical Sciences, Sunchon National University, Suncheon 57922, Republic of Korea 2Department of Chemistry and Nano Science, Ewha Womans University, Seoul 03760, Republic of Korea 3Center for Marine Biotechnology and Biomedicine, Scripps Institution of Oceanography, University of California, San Diego, La Jolla, CA 92093-0204, USA Received: December 19, 2016 Revised: December 27, 2016 Two piloquinone derivatives isolated from Streptomyces sp. CNQ-027 were tested for the Accepted: January 4, 2017 inhibitory activities of two isoforms of monoamine oxidase (MAO), which catalyzes monoamine neurotransmitters. The piloquinone 4,7-dihydroxy-3-methyl-2-(4-methyl-1- oxopentyl)-6H-dibenzo[b,d]pyran-6-one (1) was found to be a highly potent inhibitor of First published online human MAO-B, with an IC50 value of 1.21 µM; in addition, it was found to be highly effective January 9, 2017 against MAO-A, with an IC50 value of 6.47 µM. Compound 1 was selective, but not extremely *Corresponding author so, for MAO-B compared with MAO-A, with a selectivity index value of 5.35. Compound 1,8- Phone: +82-61-750-3751; dihydroxy-2-methyl-3-(4-methyl-1-oxopentyl)-9,10-phenanthrenedione (2) was moderately Fax: +82-61-750-3708; effective for the inhibition of MAO-B (IC = 14.50 µM) but not for MAO-A (IC > 80 µM).