Study on Suitability of Groundwater for Irrigation Purpose in Parambikulam Aliyar Project Area, India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNITED NATIONS NATIONS UNIES 2 July 2002 Dear Mr. Kaarthikeyan, I
UNITED NATIONS NATIONS UNIES EXECUTIVE OFFICE OF THE SECRETARY-GENERAL CABINET PU SECRETAIRE GENERAL Rm. S-3840A - Tel. (212) 963-5495 -- FAX (212) 963-1185 2 July 2002 Dear Mr. Kaarthikeyan, I write in reply to your recent letter to the Secretary-General in which you invite him to deliver a message of advice, greetings and blessings at the World Congress on Global Vision and Strategies for Peace, Non-Violence and Harmony that will be held from August 10th to August 14th in Aliyar. I trust you will understand that the intense pressure on the Secretary-General's schedule prevents him from making a special statement. Nonetheless, I should like to send you a copy of the message the Secretary-General delivered on occasion of the "Decade for a Culture of Peace and Non-Violence for the Children of the World." You may wish to read or print an excerpt of this message, which is relevant to the theme of your meeting. On behalf of the Secretary-General, I should like to extend his thanks to you, and his best wishes for the success of your congress. Yours sincerely, Gillian Martin Sorensen Assistant Secretary-General for External Relations Mr. D.R. Kaarthikeyan Co-Chairman Organizing Committee Cl-1, Humayun Road, New Delhi 110 003, INDIA UNITED NATIONS WSff NATIONS UNIES THE SECRETARY-GENERAL MESSAGE ON THE INTERNATIONAL DECADE FOR THE CULTURE OF PEACE AND NON-VIOLENCE FOR THE CHILDREN OF THE WORLD The International Decade for the Culture of Peace and Non-Violence for the Children of the World is an invitation to the entire international community to come together in the struggle for peace. -
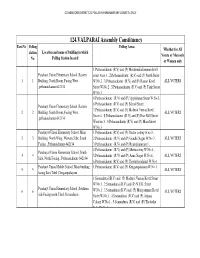
124.VALPARAI Assembly Constituency Part.No Polling Polling Areas Whether for All Station Location and Name of Building in Which Voters Or Men Only No
COIMBATORE DISTRICT 21 POLLACHI PARLIMENTARY CONSTITUENCY 124.VALPARAI Assembly Constituency Part.No Polling Polling Areas Whether for All station Location and name of building in which Voters or Men only No. Polling Station located or Women only 1.Pethanaickanur (R.V) and (P) Mondimakaliamman kovil Panchayt Union Elementary School ,Eastern street w.no 1 , 2.Pethanaickanur (R.V) and (P) North Street 1 1 Building, North Room, Facing West. W.No.2 , 3.Pethanaickanur (R.V) and (P) Ramar Kovil ALL VOTERS ,pethanaickanur-642134 Street W.No.2 , 5.Pethanaickanur (R.V) and (P) Tank Street W.No.3 , 4.Pethanaickanur (R.V) and (P) Uppukinnar Street W.No.3, 6.Pethanaickanur (R.V) and (P) School Street , Panchayt Union Elementary School ,Eastern 7.Pethanaickanur (R.V) and (P) Madurai Veeran Kovil 2 2 Building, South Room, Facing West. ALL VOTERS Street 4 , 8.Pethanaickanur (R.V) and (P) Rice Mill Street ,pethanaickanur-642134 Ward no 3 , 9.Pethanaickanur (R.V) and (P) Main Street W.No.3 Panchayat Union Elementary School ,Main 1.Pethanaickanur (R.V) and (P) Doctor colony w.no 3 , 3 3 Building. North Wing, Western Side, South 2.Pethanaickanur (R.V) and (P) Gandhi Nagar W.No 3 , ALL VOTERS Facing. ,Pethanaickanur-642134 3.Pethanaickanur (R.V) and (P) Rajendrapuram 1 , 4.Pethanaickanur (R.V) and (P) Mettucolony W.No 4 , Panchayat Union Elementary School ,South 4 4 5.Pethanaickanur (R.V) and (P) Anna Nagar W.No 4 , ALL VOTERS Side, North Facing. ,Pethanaickanur-642134 6.Pethanaickanur (R.V) and (P) Thottathusalaigal W.No 1 Panchayt Union Middle School ,Main building 1.Pethanaickanur (R.V) and (P) Kengampalayam W.No 1 5 5 ALL VOTERS facing East,Tiled ,Gengampalayam 1.Somandurai (R.V) and (P) Madurai Veeran Kovil Street W.No.1 , 2.Somandurai (R.V) and (P) N.S.K. -

DISTRICT SURVEY REPORT for GRAVEL and BRICK EARTH COIMBATORE DISTRICT Page Chapter Content No
DISTRICT SURVEY REPORT FOR GRAVEL AND BRICK EARTH COIMBATORE DISTRICT (Prepared as per Gazette Notification S.O.3611 (E) Dated 25.07.2018 of Ministry of Environment, Forest and Climate Change MoEF& CC) MAY 2019 DISTRICT SURVEY REPORT FOR GRAVEL AND BRICK EARTH COIMBATORE DISTRICT Page Chapter Content No. 1. Introduction 1 2. Overview of Mining Activity in the District 4 3. General Profile of the District 4 4. Geology of the District 8 5. Drainage of Irrigation pattern 11 6. Land Utilisation Pattern in the District: Forest, Agricultural, 13 Horticultural, Mining etc., 7. Surface Water and Ground Water scenario of the District 15 8. Rainfall of the District and Climatic conditions 16 9. a) Occurrence of Gravel and Brick Earth in the Coimbatore District 16 b) Details of the mining/quarry leases in the District as per the following 17 format Details of Seigniorage feeReceived in last three years (2016-17 to 2018- 10. 18 19) 11. Details of Production in last three years (2016-17 to 2018-19) 18 12. Mineral map of the District 19 List of Letter of Intent (LOI) Holders in the District along with its 13. 20 validity as per the following format 14. Total mineral reserves available in the District 20 15. Quality/ Grade of Mineral available in the District 20 16. Use of Mineral 21 17. Demand and supply of the Mineral in the last three years 21 18. Mining leases marked on the Map of the District 22 Details of the area of where there is a cluster of Mining Leases 19. -
Name Address
NEWS 1. Rev.M.SAM JAYAKUMAR CONSECREATED AS A DIOCESAN BISHOP FOR COIMBATORE DISTRICT 2. REV.A.G.SOUNDARRAJAN CONSECREATED AS A DIOCESAN BISHOP FOR SALEM DISTRICT. 3. REV.DR.P.NEESAN APPOINTED AS A STATE PRESIDENT. 4. RT.REV.DR.J.LAWRANCE GUNASEKARAN APPOINTED AS AN INTL- MISSION DIOCESE DIRECTOR. 5. Rt. REV.SAM S.PETER APPOINTED AS SYNOD GENERAL SECRETARY. 6. Rev. Dr. R. Samuel Raj, Consecreated as a Coimbatore city Bishop & Bishop College Secretary 7. Rev. Dr. I.P Baskaran, Coimbatore City Associated general Bishop ORDAINED REVEREND Reg No, NAME ADDRESS 1 REV. CANON.SAM S. PETER NO1/14 SHANMUGAPURAM EXTENSION 9894548055 VIII TH STREET,THIUVOTTIYUR,CHENNAI PIN:6000019. 2 REV.DR., P.K.SAM SUNDAR NO13A/2 IYYAPILLAI GARDEN, 9444309337 1 STREET,KALADIPETTAI,THIRUVOTTIYUR CHENNAI-600019. 3 REV.D.THIRIPURASUNDARI NO1/14 SHANMUGAPURAM EXTENSION 9566240903 VIII TH STREET,THIUVOTTIYUR,CHENNAI PIN:6000019. 4 EVA.V.SIGAMANI N0:55,BAJANAI KOIL STREET, PERIYAKAVANAM,PONNERI. PIN:601204. 5 REV.M.SIGAMANI N0:486 ANNAI THERASA STREET, 9789554771 PERIYAR NAGAR,ARANVOYAL P.O. THIRUVALLUR DIST, PIN:602025. 6 REV.DR.P.NEESAN NO: PALAYAKARA STREET, 8428690088 PERIYAPALAYAM, UTHUKOTTAI T.K.THIRUVALLUR DIST, PIN:601102. 7 REV.DR.,I.MERZ RETHNAPAUL NO:4/54 SELAS,KATTARY,P.O. 9443575224 COONOOR MAIN ROAD, COONOOR,NILIGIRI DISTRICT, PIN:643213. 8 REV.T.ALAGESAN NO:5/839 VALLUVAR NAGAR, OTTUPATTARAI,COONOOR,NILIGIRI. PIN:643105. 9 REV.A.SURESHKUMAR NO:41DARLINGTON BRIDGE, COONOOR, NILIGIRI DIST., PIN:643101 10 REV.K.RAMESH DAVID NO:7/32A.JOTHI NAGAR, 9626342296 ELLANALLI,COONOOR T.K, NILIGIRI DISTRICT, PIN:643101 11 EVA.K.RANI NO:16/A TENTHILL,COONOOLR, NILIGIRI DISTRICT, PIN:643102 12 REV.S.PREAM KUMAR NO:11/350 PRAGASAPURAM 9842878216 KETTY P.O. -

Tribes of the Anamalais
NCF Technical Report No. 16 TRIBES OF THE ANAMALAIS LIVELIHOOD AND RESOURCE‐USE PATTERNS OF MANISH CHANDI COMMUNITIES IN THE RAINFORESTS OF THE INDIRA GANDHI WILDLIFE SANCTUARY AND VALPARAI PLATEAU TRIBES OF THE ANAMALAIS LIVELIHOOD AND RESOURCE‐USE PATTERNS OF COMMUNITIES IN THE RAINFORESTS OF THE INDIRA GANDHI WILDLIFE ANCTUARY AND ALPARAI PLATEAU S V ANISH HANDI M C 3076/5, IV Cross, Gokulam Park , Mysore 570 002, INDIA Web: www.ncf‐india.org; E‐mail: ncf@ncf‐india.org Tel.: +91 821 2515601; Fax +91 821 2513822 Chandi, M. 2008. Tribes of the Anamalais: livelihood and resource-use patterns of communities in the rainforests of the Indira Gandhi Wildlife Sanctuary and Valparai plateau. NCF Technical Report No. 16, Nature Conservation Foundation, Mysore. Cover photographs (Photos by the author) Front cover: View of Kallarkudi, a Kadar settlement in the Indira Gandhi Wildlife Sanctuary, as seen from Udumanparai. Back cover: Thangaraj and his family processing coffee berries at Nedungkundru, a Kadar settlement (left) and Srinivasan from Koomati, a Malai Malasar settlement, demonstrating climbing a tree pegged earlier for honey collection (right). CONTENTS Acknowledgements 1 Summary 2 1. Background 3 2. Identity and Change 10 3. Livelihood and Resource Use 36 4. Infrastructure and Demography 56 5. Conclusions 65 6. References and Readings 72 7. Annexures 77 ACKNOWLEDGEMENTS I had just returned from the Andaman Islands when during the course of a conversation over lunch Janaki asked if I knew anybody who would be interested in profiling indigenous communities in the Anamalais. I had only fleetingly heard of this region though I was keen to know more. -

Project Work Guidelines 15.11.2010
REPORT ON INDUSTRIAL VISIT TO PAP ON 13.08.2010 REPORT ON INDUSTRIAL VISIT TO PARAMBIKULAM ALIYAR PROJECT (PAP) on 13 - 8 - 2010 by III. B. E. Civil Engineering (2008 – 2012 batch) Dr. Mahalingam College of Engineering and Technology Pollachi – 642 003. Page 1 of 20 REPORT ON INDUSTRIAL VISIT TO PAP ON 13.08.2010 Page 2 of 20 REPORT ON INDUSTRIAL VISIT TO PAP ON 13.08.2010 TABLE OF CONTENTS 1 INTRODUCTION:....................................................................................................................................3 2 AIM OF THE PROJECT:........................................................................................................................3 3 RESERVOIRS...........................................................................................................................................3 3.1 UPPER NIRAR WEIR:- ....................................................................................................................................... 3 3.2 LOWER NIRAR DAM:-........................................................................................................................................ 3 3.3 SHOLAYAR RESERVOIR:-................................................................................................................................. 3 3.4 ANAMALAYAR DIVERSION WORK:- ................................................................................................................. 3 3.5 PARAMBIKULAM RESERVIOR :-..................................................................................................................... -

Research Article SPATIAL and TEMPORAL VARIABILITY of PRECIPITATION CONCENTRATION INDEX (PCI) and RAINFALL TREND for PARAMBIKULAMALIYAR BASIN AREA TAMIL NADU
International Journal of Agriculture Sciences ISSN: 0975-3710 & E-ISSN: 0975-9107, Volume 10, Issue 12, 2018, pp.-6451-6454. Available online at https://www.bioinfopublication.org/jouarchive.php?opt=&jouid=BPJ0000217 Research Article SPATIAL AND TEMPORAL VARIABILITY OF PRECIPITATION CONCENTRATION INDEX (PCI) AND RAINFALL TREND FOR PARAMBIKULAMALIYAR BASIN AREA TAMIL NADU SATHYA L.* AND SANTHANABOSU S. Department of Soil and Water Conservation Engineering, Agricultural Engineering, College and Research Institute, Tiruchirappalli, 621712, Tamil Nadu Agricultural University, Coimbatore, 641003, Tamil Nadu, India *Corresponding Author: Email- [email protected] Received: June 19, 2018; Revised: June 25, 2018; Accepted: June 26, 2018; Published: June 30, 2018 Abstract: This paper presents the trend in the annual rainfall and its distribution over Parambikulam Aliyar basin of Tamil Nadu determined using 30 years (1988-2008) monthly rainfall data at 28 rain-gauge stations. The trend analysis was done by using Mann-Kendall test. The results indicated increasing trends in annual rainfall. The mean Precipitation Concentration Index of Valaiyar sub basin and Palar sub basin indicates strongly irregular monthly distribution of annual rainfall. The mean Precipitation Concentration Index of Aliyar sub basin and Sholaiyar sub basin indicates irrugular monthly distribution of annual rainfall. The temporal pattern of rainfall trends analyzed in this study is a basic and important requirement for agricultural planning and management of water resources. Keywords: Precipitation Concentration Index, Parambikulam Aliyar basin, Monthly distribution, Mann Kendal Analysis Citation: Sathya L. and Santhanabosu S. (2018) Spatial and Temporal Variability of Precipitation Concentration Index (Pci) and Rainfall Trend for Parambikulam Aliyar Basin Area Tamil Nadu. International Journal of Agriculture Sciences, ISSN: 0975-3710 & E-ISSN: 0975-9107, Volume 10, Issue 12, pp.- 6451-6454. -

New Letter-August-17-Final
NEWS LETTER NEWS LETTER Free Circulation Vol. 4 Issue : 1 shows that it is more closely related to the sheep. Its close relative is Himalayan Tahr . Nilgiri ss tahr is distributed in Upper Nilgiris, Coimbatore Division, Anamalai Tiger Reserve, Kodaikanal ii Wildlife sanctuary, Theni Forest Division, Srivilliputhur Grizzled squirrel wildlife sanctuary and laa Kanyakumari Forest Division in Tamil Nadu and Eravikulam National park, Chinnar Wildlife aal sanctuary of Kerala. It inhabits the open montane grasslands and recent observations reveals that they are also found in dry habitats of Amaravathi and Udumalpet ranges and in between mmAnamalai Tiger Conservation Foundation rd th a 3 and 9 hairpin bend along the Aliyar-Valparai Road. ATR has second largest population of a n Tamil Nadu Trust, Pollachi. Nilgiri Tahr in Tamil Nadu after Mukuruthi National park. AAn A large Cycas Tree located A big Cycas circinalis tree was located at Vellimudi, Ulandy range by Thiru V. Ganesan I.F.S., Field Director, ATR and his team during his field visit on 11th December 2016. This plant belongs to the family Cycadaceae and it is endemic to India. C circinalis is a palm like evergreen plant belonging to Gymnosperm group of plants found in the tropical forests. It is a medium sized suckering plant growing up to 3-5 meters tall. They have not undergone much change right from its origin during Mesozoic Era and hence it is considered as living fossil. It is a dioecious plant. It takes nearly 10 years for the plant to reach sexual maturity. Cycas trees are threatened worldwide and all species in this family are listed in IUCN Red list. -

Present Status of the Water Quality Parameters of the Aliyar Dam, Pollachi, Coimbatore District, Tamil Nadu
International Journal of Pharmacy and Biological Sciences TM ISSN: 2321-3272 (Print), ISSN: 2230-7605 (Online) IJPBSTM | Volume 8 | Issue 3 | JUL-SEPT | 2018 | 1012-1024 Research Article | Biological Sciences | Open Access | MCI Approved| |UGC Approved Journal | PRESENT STATUS OF THE WATER QUALITY PARAMETERS OF THE ALIYAR DAM, POLLACHI, COIMBATORE DISTRICT, TAMIL NADU C. Karthik* and M. Lekshmanaswamy PG and Research Department of Zoology, Kongunadu Arts and Science College (Autonomous), Coimbatore – 641 029, Tamil Nadu, India. *Corresponding Author Email: [email protected] ABSTRACT In the present study, the water samples were collected from three different locations of Aliyar Dam, Pollachi Taluk, Coimbatore District, Tamil Nadu, India for physico-chemical analysis. The laboratory tests of the collected water samples were carried out for the analysis of various parameters such as temperature, pH, Electrical conductivity, Suspended Solids, Dissolved Solids, Total Solids, Total Alkalinity, Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD), Fluoride, Calcium, Magnesium, Sulphate, Phosphate, Chloride, Nitrate, Iron and Carbonates. The methods employed for analysis as per standard methods recommended by APHA, WHO. The results obtained were compared with BIS standards and the parameters showing slight variations than standards that affects the water quality. These variations may be influenced by sample locations, time of sampling and activities carried out around the dam. Hence, it is recommended that the water can be used for drinking purposes with proper treatment and regular water quality monitoring. KEY WORDS Aliyar dam, APHA, BIS, drinking, irrigation, physico-chemical parameters and water quality INTRODUCTION cooling and heating), (iv) agricultural purposes Water is essential source and basis for survival of all (irrigation) and (v) Power production (hydro power and living organisms. -
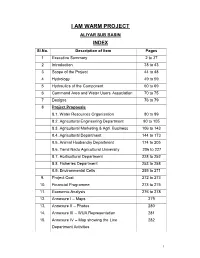
ALIYAR SUB BASIN INDEX Sl.No
I AM WARM PROJECT ALIYAR SUB BASIN INDEX Sl.No. Description of Item Pages 1 Executive Summary 2 to 37 2 Introduction 38 to 43 3 Scope of the Project 44 to 48 4 Hydrology 49 to 59 5 Hydraulics of the Component 60 to 69 6 Command Area and Water Users Association 70 to 75 7 Designs 76 to 79 8 Project Proposals 8.1. Water Resources Organisation 80 to 89 8.2. Agricultural Engineering Department 90 to 105 8.3. Agricultural Marketing & Agri. Business 106 to 143 8.4. Agricultural Department 144 to 173 8.5. Animal Husbandry Department 174 to 205 8.6. Tamil Nadu Agricultural University 206 to 227 8.7. Horticultural Department 228 to 252 8.8. Fisheries Department 253 to 258 8.9. Environmental Cells 259 to 271 9. Project Cost 272 to 273 10. Financial Programme 273 to 275 11. Economic Analysis 276 to 278 12. Annexure I – Maps 279 13. Annexure II – Photos 280 14. Annexure III – WUA Representation 281 15. Annexure IV – Map showing the Line 282 Department Activities 1 EXECUTIVE SUMMARY 2 Executive Summary Introduction The Parambikulam Aliyar Project is an interstate Water Resources Development project carried out jointly by the states of Tamilnadu and Kerala . The objective of the development is harnessing th e waters of the Bharathapuzha, the Chalakudi and the Periyar basins for irrigation and power production in both the states. It accomplishes the diversion and integration of eight west flowing rivers, six in the Anaimalai hills and two in the plains for t he benefit of the drought prone areas in the Coimbatore and Erode districts of Tamilnadu state and Palghat District of Kerala State. -

Temporal Variation of Rainfall Trends in Parambikulam Aliyar Sub Basin, Tamil Nadu R
International Journal of Agriculture, Environment and Biotechnology Citation: IJAEB: 8(1): 37-48 March 2015 DOI Number: 10.5958/2230-732X.2015.00005.4 ©2015 New Delhi Publishers. All rights reserved AGRICULTURE ENGINEERING 5 (OK) Temporal variation of rainfall trends in parambikulam aliyar sub basin, Tamil Nadu R. Vinoth Kumar1* and I. Muthuchamy2 1,2*Tamil Nadu Agricultural University, Department of Soil and Water Conservation Engineering, Agricultural Engineering College and Research Institute, Coimbatore – 641 003, Tamil Nadu, India. *Corresponding author: [email protected] Paper No. 289 Received: 18 November 2014 Accepted: 10 February 2014 Published: 24 April 2015 ABSTRACT Identification of temporal variation of rainfall trends provides useful information for sustainable planning and management of water resources in a river basin particularly during flood and drought periods. The present study was conducted to determine trends in the annual and seasonal total rainfall over Parambikulam Aliyar sub basin of Tamil Nadu using 30 years (1982-2012) monthly rainfall data at eight rain-gauge stations. The procedure is based on the nonparametric Mann-Kendall test for the trend and the nonparametric Sen’s method for the magnitude of the trend. The maximum increase in rainfall was found at Chinnakallar with an annual rainfall of 90.51 mm/year and the maximum reduction in rainfall of -8.60 mm/year was found at Topslip during South West monsoon. Significant positive trend were observed at Weaverly station during North East monsoon rainfall series and significant negative trend has been noticed in the South West monsoon of Topslip. Highlights • Positive trends were observed in most of the rain-gauge stations for all rainfall series except South West monsoon season. -

Macaca Silenus) in Annamalai Hills, Western Ghats, Southern India
ISSN: 2688-3856 Research Article JOJ Wildl Biodivers Copyright © All rights are reserved by Moorthi Mahaly Volume 2 Issue 4- July 2020 Population structure and Food preference of Lion-tailed Macaques (Macaca silenus) in Annamalai Hills, Western Ghats, Southern India Ravichandran Srimathi and Moorthi Mahaly* Department of Zoology and Wildlife Biology, AVC College (Autonomous), (Affiliated to Bharathidasan University), Tamil Nadu, India Submission: June 02, 2020; Published: July 31, 2020 *Corresponding author: Bharathidasan University) Mayiladuthurai –609 305, Tamil Nadu, India Moorthi Mahaly, Research Department of Zoology and Wildlife Biology, AVC College (Autonomous), (Affiliated to Abstract The Lion-tailed Macaque (Macaca silenus), endemic to the evergreen forests of the Western Ghats in Southern India, is endangered. The present study focuses on population and food preference of Lion-tailed Macaque (LTM) in the Anaimalai Hills, Western Ghats. The tropical rainforest of Anaimalai Hills, State of Tamil Nadu was surveyed in April-2018 to May-2018. The surveyed Regions (R) of Anaimalai Hills such as R1-Puthuthotam Estate (PT), R2-Kavarkal Estate (KE), R3-Wayverlay Estate (WE), R4-Water Falls Estate (WF) and R5-Attakatti (AK), these all are the major LTM distributed area. The LTM population was estimated by the Buckland and Thomas line transects method and one zero samplings methods were used by (Altmann) to assess the feeding activity. Population estimation in this study obtained a total of 53 sightings of lion-tailed Macaque (Macaca silenus) groups. The estimated number of troops in the region was 34. The estimated minimum population size 21.16 and SD of the population is 5.48.