University of Florida Thesis Or Dissertation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Redalyc.Functional Response of the Predatory Mite Chileseius Camposi
Revista de Biología Tropical ISSN: 0034-7744 [email protected] Universidad de Costa Rica Costa Rica Sepúlveda, Francisco; Carrillo, Roberto Functional response of the predatory mite Chileseius camposi (Acarina: Phytoseiidae) on densities of it prey, Panonychus ulmi (Acarina: Tetranychidae) Revista de Biología Tropical, vol. 56, núm. 3, septiembre, 2008, pp. 1255-1260 Universidad de Costa Rica San Pedro de Montes de Oca, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44918834022 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Functional response of the predatory mite Chileseius camposi (Acarina: Phytoseiidae) on densities of it prey, Panonychus ulmi (Acarina: Tetranychidae) Francisco Sepúlveda1 & Roberto Carrillo1 1. Instituto de Producción y Sanidad Vegetal, Universidad Austral de Chile,Casilla 567, Valdivia, Chile; [email protected] Received 24-IV-2007. Corrected 01-II-2008. Accepted 31-VII-2008. Abstract: The functional response of the phytoseiid predator Chileseius camposi González y Schuster, 1962 (Acarina:Phytoseiidae) on densities of its prey Panonychus ulmi (Koch, 1836) was evaluated under controlled temperature (20 ± 2 ºC),, relative humidity (75 ± 15%) and photoperiod (16:8h L:D). A functional type II response was displayed (Holling’s disk). Holling, Wolf transformation and Rogers models gave similar values for estimating parameters of the Holling’s disk equation; however, estimates produced by the Livdahl and Stiven model were higher. Values of attack rate and handling time can be considered within the normal range for phy- toseiid generalists. -

Mesostigmata No
13 (1) · 2013 Christian, A. & K. Franke Mesostigmata No. 24 ............................................................................................................................................................................. 1 – 32 Acarological literature Publications 2013 ........................................................................................................................................................................................... 1 Publications 2012 ........................................................................................................................................................................................... 6 Publications, additions 2011 ....................................................................................................................................................................... 14 Publications, additions 2010 ....................................................................................................................................................................... 15 Publications, additions 2009 ....................................................................................................................................................................... 16 Publications, additions 2008 ....................................................................................................................................................................... 16 Nomina nova New species ................................................................................................................................................................................................ -

DIET CHOICE and HABITAT STRUCTURE MEDIATE COEXISTENCE of PREDATORY MITES on Jatropha Curcas
RENATA VIEIRA MARQUES DIET CHOICE AND HABITAT STRUCTURE MEDIATE COEXISTENCE OF PREDATORY MITES ON Jatropha curcas Tese apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa de Pós-Graduação em Entomologia, para obtenção do título de Doctor Scientiae. VIÇOSA MINAS GERAIS – BRASIL 2019 Ficha catalográfica preparada pela Biblioteca Central da Universidade Federal de Viçosa - Câmpus Viçosa T Marques, Renata Vieira, 1989- M357d Diet choice and habitat structure mediate coexistence of 2019 predatory mites on Jatropha curcas / Renata Vieira Marques. – Viçosa, MG, 2019. 100 f. : il. ; 29 cm. Texto em inglês. Orientador: Angelo Pallini Filho. Tese (doutorado) - Universidade Federal de Viçosa. Inclui bibliografia. 1. Ácaros - Controle biológico. 2. Euseius concordis. 3. Iphiseiodes zulluagai. 4. Animais predadores. 5. Dieta. I. Universidade Federal de Viçosa. Departamento de Entomologia. Programa de Pós-Graduação em Entomologia. II. Título. CDD 22. ed. 595.42 Scanned by CamScanner AGRADECIMENTOS Primeiramente gostaria de agradecer a Deus pelo dom da vida e por me permitir seguir forte durante toda a jornada do doutoramento. Aos meus Pais que sempre me apoiaram e me mostraram que eu era capaz de realizar meus sonhos e muito mais. Sempre foram minha base e me apoiaram nos momentos mais difíceis. Mas também se fizeram presentes nos momentos de conquistas. Ao meu marido Victor Vidal faço um agradecimento muito especial, por sempre me incentivar a crescer e conseguir alcançar meus objetivos. Com toda paciência permaneceu ao meu lado nos momentos difíceis. A minha pequena Manuella, gostaria de dizer que junto com seu nascimento nasceu uma mulher mais dedicada, esforçada, pontual, organizada, capaz de conseguir alcançar seus sonhos. -

Mesostigmata No
16 (1) · 2016 Christian, A. & K. Franke Mesostigmata No. 27 ............................................................................................................................................................................. 1 – 41 Acarological literature .................................................................................................................................................... 1 Publications 2016 ........................................................................................................................................................................................... 1 Publications 2015 ........................................................................................................................................................................................... 9 Publications, additions 2014 ....................................................................................................................................................................... 17 Publications, additions 2013 ....................................................................................................................................................................... 18 Publications, additions 2012 ....................................................................................................................................................................... 20 Publications, additions 2011 ...................................................................................................................................................................... -

Mite Fauna (Arachnida: Acari) on Peach Cultivars in Presidente Prudente, São Paulo, Brazil
Journal of Plant Studies; Vol. 1, No. 2; 2012 ISSN 1927-0461 E-ISSN 1927-047X Published by Canadian Center of Science and Education Mite Fauna (Arachnida: Acari) on Peach Cultivars in Presidente Prudente, São Paulo, Brazil Sônia Maria Nalesso Marangoni Montes1, Adalton Raga2, Aparecida Conceição Boliani3, Jeferson Luiz de Carvalho Mineiro2 & Pedro César dos Santos3 1 Sao Paulo State Agency of Technology Agribusiness-APTA, Regional Alta Sorocabana, Route Raposo Tavares km 561, Box 298, Presidente Prudente, SP 19015-970, Brazil 2 APTA- Biological Institute, Avenue Heitor Penteado km 3, Box 70 Campinas, SP 13001-970, Brazil 3 Paulist State University-UNESP, Campus de Ilha Solteira, Avenue Brasil, 56, Ilha Solteira, SP 15385-000, Brazil Correspondence: Sônia Maria Nalesso Marangoni Montes, Sao Paulo State Agency of Technology Agribusiness-APTA, Regional Alta Sorocabana Route Raposo Tavares km 561, Box 298, Presidente Prudente, SP 19015-970, Brazil. Tel: 55-18-3222-0732. E-mail: [email protected] Received: March 15, 2012 Accepted: May 20, 2012 Online Published: September 1, 2012 doi: 10.5539/jps.v1n2p173 URL: http://dx.doi.org/10.5539/jps.v1n2p173 Research supported by FAPESP (Processo nº05/55649-5) Abstract This study aimed to determine the mite diversity, population dynamics and to conduct a fauna analysis in plantations from four peach varieties established in the municipality of Presidente Prudente, SP, Brazil. The mite fauna from ‘Jóia 4’, ‘Ouromel 3’, ‘Regis’ and ‘Rei da conserva’ cultivars over the rootstock Okinawa were determined from December 2002 to February 2006. Samples composed by 72 leaves were collected fortnightly from upper, middle and lower third of each tree and four trees per cultivar. -
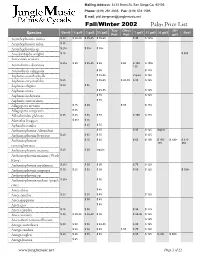
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Saving Tahina — You Can Help
July 2020 NEWSLETTER SAVING TAHINA — YOU CAN HELP PalmTalk (the public forum of the International Palm Society) is initiating a pro- gram to preserve Tahina spectabilis, an endangered palm that PalmTalk brought to the world’s attention in 2007. The discovery of this enormous, new palm gen- erated worldwide interest, but now due to its extremely limited and fragile range in Madagascar, Tahina needs our help. Please visit PalmTalk (go to www. palms.org, and click on the PalmTalk Forums tab) to see how the IPS and the Roy- al Botanic Gardens, Kew are partnering with a local village in Madagascar to help save this amazing palm. You can make a donation to the pro- ject and be a part of this unique partnership in conser- vation. Please donate today! One of the first photos posted on PalmTalk of the mysterious palm that came to be known as Tahina spectabilis and the little girl (at the time) for whom it was named, Anne-Tahina Metz. Volume 8.06 · July 2020 · Newsletter of the International Palm Society | Editor: Andy Hurwitz [email protected] Virtual hiking on Reunion Island, lowlands editionby Andy Hurwitz This article continues the “virtual hike” and tour of Reunion’s palms that began in last month’s News- letter. This month, we explore the palms and landscapes of the low to middle elevations. We begin by traveling to the windward eastern coast of the island, just south of Piton-Sainte-Rose, to ramble in Anse des Cascades. This idyllic cove is situated among groves of palm trees. The park is cool and shady. -

Hosts of Raoiella Indica Hirst (Acari: Tenuipalpidae) Native to the Brazilian Amazon
Journal of Agricultural Science; Vol. 9, No. 4; 2017 ISSN 1916-9752 E-ISSN 1916-9760 Published by Canadian Center of Science and Education Hosts of Raoiella indica Hirst (Acari: Tenuipalpidae) Native to the Brazilian Amazon Cristina A. Gómez-Moya1, Talita P. S. Lima2, Elisângela G. F. Morais2, Manoel G. C. Gondim Jr.1 3 & Gilberto J. De Moraes 1 Departamento de Agronomia, Universidade Federal Rural de Pernambuco, Recife, PE, Brazil 2 Embrapa Roraima, Boa Vista, RR, Brazil 3 Departamento de Entomologia e Acarologia, Escola Superior de Agricultura ‘Luiz de Queiroz’, Universidade de São Paulo, Piracicaba, SP, Brazil Correspondence: Cristina A. Gómez Moya, Departamento de Agronomia, Universidade Federal Rural de Pernambuco, Av. Dom Manoel de Medeiros s/n, Dois Irmãos, 52171-900, Recife, PE, Brazil. Tel: 55-81-3320-6207. E-mail: [email protected] Received: January 30, 2017 Accepted: March 7, 2017 Online Published: March 15, 2017 doi:10.5539/jas.v9n4p86 URL: https://doi.org/10.5539/jas.v9n4p86 The research is financed by Coordination for the Improvement of Higher Education Personnel (CAPES)/ Program Student-Agreement Post-Graduate (PEC-PG) for the scholarship provided to the first author. Abstract The expansion of red palm mite (RPM), Raoiella indica (Acari: Tenuipalpidae) in Brazil could impact negatively the native plant species, especially of the family Arecaceae. To determine which species could be at risk, we investigated the development and reproductive potential of R. indica on 19 plant species including 13 native species to the Brazilian Amazon (12 Arecaceae and one Heliconiaceae), and six exotic species, four Arecaceae, a Musaceae and a Zingiberaceae. -

2951 – 3000 Trinidad to British Guiana
2951 – 3000 Trinidad to British Guiana [Inside cover] Book 9 Acanthophoenix 2956 Lecythis 2963 Acrocomia 2961 Licuala 2978 Ananas 2993 Livistona 2982 Archontophoenix 2983 Lodoicea 2985 Areca 2953 2953 Mauritia 2984 “ 2954 Mokka-mokka 2997 Astrocaryum 2957 Nipa 2981 “ 2986 Pachira 2976 “ 2987 “ 3000 Asystasia 2964 Passiflora 2952 Bauhinia 2960 “ 2995 Borassus 2979 Peltogyne 2970 Bromelia 2996 Pithecolobium 2965 Caryocar 2999 Randia 2994 Citrus 2998 Samanea 2966 Copernicia 2977 Undetermined 2967 Crotolaria 2973 “ In ink:]Ochna 2971 “ 2974 “ 2972 Desmoncus 2951 “ 2989 Diospyrus 2968 “ 2990 Euterpe 2955 “ 2991 Gmelina 2969 “ 2992 Hyphaene 2980 Zingerberaciae 2958 Ixora 2975 TILLANDSIA 2996 Jacaranda 2962 2951 Demoncus minor? See Harold Loomis’ notes and photo of inflorescence. #54 Villainously spacing & hooked climbing palm reminding one of those terrible Rattan palms of the Orient. When in fruit its bunches of deep scarlet fruits are attractive. A denizen of the deep shade forest and requiring moisture. From my experience in Coconut Grove with this genus I judge it wants half shade. Collected in Arena Forest Reserve Trinidad. 2/4/32 [In pencil] Loomis Photo 220 See D. F. Photo 18454-5 2952 P. H. Dorsett [In ink] “rubra?” Passiflora Sp Wild species of passion vine collected in the outskirts of the town of Eleuthera Bluff Island of Eleuthera Bahamas. Attractive looking species useful [Break in text, in ink] 1 single plant in pot Dec. 14/32. C. F. [Continuation of text] for breeding purposes. 1-7-32 (I’d like a few seeds or plants for my passiflora collection in Coconut Grove) Passiflora sp. Wild species growing in pothole in rocky soil of Eleuthera Bluff a colored town on Eleuthera Island. -

Life Table Parameters and Consumption Rate Of
160RESEARCH CHILEAN J. AGRIC. RES. - VOL. 69 - Nº 2 - 2009 LIFE TABLE PARAMETERS AND CONSUMPTION RATE OF Cydnodromus picanus Ragusa, Amblyseius graminis Chant, AND Galendromus occidentalis (Nesbitt) ON AVOCADO RED MITE Oligonychus yothersi (McGregor) (ACARI: PHYTOSEIIDAE, TETRANYCHIDAE) Tommy Rioja S.1*, and Robinson Vargas M.2 ABSTRACT The avocado red mite Oligonychus yothersi (McGregor) is the major leaf pest in Chile’s avocado orchards. It affects leaf physiology and makes it necessary to seek new natural enemies to interact with low population densities of O. yothersi. The potentiality of three predator mites: Cydnodromus picanus Ragusa, Amblyseius graminis Chant, and Galendromus occidentalis (Nesbitt) was evaluated under laboratory conditions (27 ± 1.93 ºC, 87 ± 3.61% H.R. and 16:8 (L:D) photoperiod) on avocado leaf disks Persea americana Mill. var. Hass (Ø = 5 cm) by separately feeding eggs, immature, and adult females of O. yothersi, and registering postembryonic development, consumption, as well as life table parameters. The postembryonic development of C. picanus was significantly lower (5.46 days) compared to both A. graminis (7.33 days) and G. occidentalis (8.69 days) which were fed with immature O. yothersi. The life table parameters of C. picanus were net reproductive rate R0 = 25.41, finite rate of increase λ = 1.29, and mean generation time T = 12.46. The net intrinsic rate of increase (rm) was significantly higher for C. picanus (rm = 0.25) in contrast with G. occidentalis (rm = 0.19), while A. graminis showed rm = -0.06 indicating that its population didn’t have descendants. Under laboratory conditions, rm registered by C. -

Transmission of the Coconut Cadang-Cadang Viroid to Six Species of Palm by Inoculation with Nucleic Acid Extracts
Plant Pathology {\9H5) 34, 391-401 Transmission of the coconut cadang-cadang viroid to six species of palm by inoculation with nucleic acid extracts JULITA S. \MPER\ALand ROSEMARIE M. BAUTISTA Philippine Coconut Authority, Albav Research Center. Banao, Guinobatan, Albay, Philippines JOHN W. RANDLES Plant Pathology Department, Waite Agricultural Research Institute, University of Adelaide, South Australia Seedlings of Areca catechu (betel nut palm), Corypha elata (buri palm), Adonidia merrillii (manila palm), Elaeis guineensis (oil palm), Chrysalidocarpus lutescens (palmera) and Oreodoxa regia (royal palm) were inoculated with nucleic acid extracts from coconut palms with cadang-cadang disease. Within 2 years of inoculation, analysis using a ^-P-labelled DNA probe complementary to the coconut cadang-cadang viroid (CCCV) showed that RNA sequences identical to CCCV were present in the inoculated seedlings. Electrophoresis in polyacrylamide gels showed that these palms also contained an RNA with mobility identical to CCCW. Four to five years after inoculation, the infected palms of four species were usually stunted compared with uninoculated palms, while betel nut and palmera were not stunted. Yellowing of leaflets was observed with defined spots or mottling of the older fronds in all except betel nut palms. All infected palms showed mild or severe yellow- leaf spotting. These results widen the known host range and. hence, the potential number of viroid reservoir species in the field. INTRODUCTION examined. The presence of alternative plant The viroid associated with cadang-cadang infec- reservoirs in the field is one important aspect tion of coconut (Cocos nucifera L.) (Randies. and studies are in progress on the identification 1975) has been shown to cause the disease when of additional species that are susceptible to inoculated to coconut palm (Zelazny et al. -

On Bactrocera Zonata Eggs (Diptera: Tephritidae) As a Factitious Food
Acta Phytopathologica et Entomologica Hungarica 51 (1), pp. 123–132 (2016) DOI: 10.1556/038.51.2016.1.11 Performance of Five Species of Phytoseiid Mites (Acari: Phytoseiidae) on Bactrocera zonata Eggs (Diptera: Tephritidae) as a Factitious Food F. M. MOMEN1* , ABD-ELRADY K. NASR1, ABD-ELSATAR M. METWALLY2, 1 1 Y. A. MAHMOUD and K. M. SALEH 1Pests and Plant Protection Department, National Research Centre (NRC), 31 El-Bohoth Street, 12311 Dokki, Cairo, Egypt 2Department of Agricultural Zoology and Nematology, Faculty of Agriculture, Al-Azhar University, Cairo, Egypt (Received: 7 September 2015; accepted: 3 November 2015) Development, survival and reproduction of the generalist predatory mites, Amblyseius largoen- sis (Muma), Neoseiulus barkeri (Hughes), Typhlodromips swirskii (Athias-Henriot), Proprioseiopsis kadii (El-Halawany and Abdel-Samad) and Cydnosus negevi (Swirski and Amitai) were assessed when fed on eggs of Bactrocera zonata (Saunders) (Diptera: Tephritidae) as a factitious food. For N. barkeri and P. kadii, the development was faster, while the reproduction was higher in N. barkeri and A. largoensis than for P. kadii. Survival of immatures of T. swirskii and C. negevi was low on eggs of B. zonata and all failed to develop be- yond the protonymphal stage. A total of 35.4, 31.2 and 19.6 eggs per female, respectively, were obtained when N. barkeri, A. lar- goensis and P. kadii were fed B. zonata eggs. A diet of the peach fruit fly eggs provided the longest female longevity and highest mean total fecundity, which resulted in the highest net reproductive rate (Ro=34.61 and 32.78) and doubling time (DT=1.53 and 1.60) for N.