Peer-Reviewed Publications 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Invasive Seaweed Enhances Recruitment of a Native Bivalve: Roles of Refuge from Predation and the Habitat Choice of Recruits
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 177–185, 2006 Published August 3 Mar Ecol Prog Ser Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits Paul E. Gribben1,*, Jeffrey T. Wright2 1Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, New South Wales 2052, Australia 2Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, New South Wales 2522, Australia ABSTRACT: Invasive species may have a range of negative effects on native species in the region invaded. The invasive green alga Caulerpa taxifolia has invaded several temperate regions world- wide and now occurs in 9 estuaries in temperate eastern Australia. Despite the threat posed by C. taxifolia, virtually nothing is known of its effects on native estuarine infauna. In the present study, we investigated the distribution and abundance, habitat choice and predation of recruits (post-set juveniles) of the native Sydney cockle Anadara trapezia at 2 sites invaded by C. taxifolia in Lake Conjola, New South Wales, Australia. Recruitment of A. trapezia was significantly higher in C. taxi- folia (both with sparse [30%] and with dense [100%] cover) than in Zostera capricorni and bare sed- iment. Up to 680 recruits m–2 were observed in C. taxifolia, with the highest recruit densities occur- ring at intermediate C. taxifolia densities. However, in habitat choice experiments, recruits showed no preference for C. taxifolia over the seagrasses Z. capricorni and Halophila ovalis, but a strong pref- erence for adult A. trapezia over all macrophytes when A. trapezia were included as treatments in experiments. -
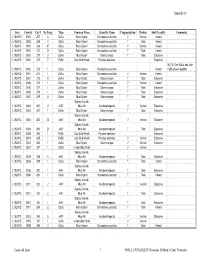
Copy of Appendix 5 Catalogue
Appendix 5.7 Area Context Cat # No.Frags Type Common Name Scientific Name Fragmentation Portion Shell Locality Comments L102W/D 3992 267 6 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 268 4 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 269 47 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 270 27 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 271 3 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/D 3992 272 7 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine NOTE: One SaCu and One L102W/D 3992 273 1 SaCu Rock Oyster Saccostrea cucullata Varied PyEb joined together L102W/D 3961 274 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3961 275 6 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3986 276 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/C 3456 277 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3553 278 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3557 279 2 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 280 2 AnTr Mud Ark Anadara trapezia V Ventral Estuarine L102W/C 3605 281 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 282 22 AnTr Mud Ark Anadara trapezia V Ventral Estuarine Sydney Cockle, L102W/C 3684 283 23 AnTr Mud Ark Anadara trapezia T Total Estuarine L102W/C 3684 284 1 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine L102W/C 3514 285 1 PyEb Club Mud Whelk Pyrazus ebeninus V Ventral Estuarine L102W/C 3514 286 1 OsAn Mud -

E Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary
!e Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary Jessica Reeves & John Buckeridge Published by: Greypath Productions Marine Care Ricketts Point PO Box 7356, Beaumaris 3193 Copyright © 2012 Marine Care Ricketts Point !is work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission of the publisher. Photographs remain copyright of the individual photographers listed. ISBN 978-0-9804483-5-1 Designed and typeset by Anthony Bright Edited by Alison Vaughan Printed by Hawker Brownlow Education Cheltenham, Victoria Cover photo: Rocky reef habitat at Ricketts Point Marine Sanctuary, David Reinhard Contents Introduction v Visiting the Sanctuary vii How to use this book viii Warning viii Habitat ix Depth x Distribution x Abundance xi Reference xi A note on nomenclature xii Acknowledgements xii Species descriptions 1 Algal key 116 Marine invertebrate key 116 Glossary 118 Further reading 120 Index 122 iii Figure 1: Ricketts Point Marine Sanctuary. !e intertidal zone rocky shore platform dominated by the brown alga Hormosira banksii. Photograph: John Buckeridge. iv Introduction Most Australians live near the sea – it is part of our national psyche. We exercise in it, explore it, relax by it, "sh in it – some even paint it – but most of us simply enjoy its changing modes and its fascinating beauty. Ricketts Point Marine Sanctuary comprises 115 hectares of protected marine environment, located o# Beaumaris in Melbourne’s southeast ("gs 1–2). !e sanctuary includes the coastal waters from Table Rock Point to Quiet Corner, from the high tide mark to approximately 400 metres o#shore. -

New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2013): 6.14 | Impact Factor (2013): 4.438 New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India Souji.S1, Tresa Radhakrishnan2 Department of Aquatic Biology and Fisheries, University of Kerala, Thiruvananthapuram-695 581, Kariyavattom, Kerala, India Abstract: Arcacea family is economically important group of animals. Most of the species in this family are misplaced into invalid subgenera and Indian arcids are wanted a revision in systematic positon. In the case of Arcidae family; all of the species are treated under Anadara as main genera, however, some authors considered that the Tegillarca genus is only a sub genus of Arcidae family. Anadara is the commercially important genus of bivalves of Arcidae family. These two genera are confused by many taxonomists and some considered that the morphometric changes of Tegillarca are only the habitual adaptation. But the collected samples from the same habitat from the southern part of India is clearly demarked the distinction between Anadara species and Tegillarca species. In this paper the differences between these two genera are illustrated with the help of specimens from the same habitat and with the help of taxonomic literature of these genera. Species level classification was done based on the morphometric characters like peculiarities of (i) periostracum, (ii) cardinal area, (iii) umbo, (iv) adductor muscle scar and (v) pallial line. The specimens were collected from Neendakara, Vizhinjam and Kovalam along with the south west coast and Thiruchendur in Tamil Nadu, south east coast of India. -

Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora)
Gulf of Mexico Science Volume 34 Article 4 Number 1 Number 1/2 (Combined Issue) 2018 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution Martha Reguero Universidad Nacional Autónoma de México Andrea Raz-Guzmán Universidad Nacional Autónoma de México DOI: 10.18785/goms.3401.04 Follow this and additional works at: https://aquila.usm.edu/goms Recommended Citation Reguero, M. and A. Raz-Guzmán. 2018. Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution. Gulf of Mexico Science 34 (1). Retrieved from https://aquila.usm.edu/goms/vol34/iss1/4 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf of Mexico Science by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Reguero and Raz-Guzmán: Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Lagu Gulf of Mexico Science, 2018(1), pp. 32–55 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution MARTHA REGUERO AND ANDREA RAZ-GUZMA´ N Molluscs were collected in Laguna Madre from seagrass beds, macroalgae, and bare substrates with a Renfro beam net and an otter trawl. The species list includes 96 species and 48 families. Six species are dominant (Bittiolum varium, Costoanachis semiplicata, Brachidontes exustus, Crassostrea virginica, Chione cancellata, and Mulinia lateralis) and 25 are commercially important (e.g., Strombus alatus, Busycoarctum coarctatum, Triplofusus giganteus, Anadara transversa, Noetia ponderosa, Brachidontes exustus, Crassostrea virginica, Argopecten irradians, Argopecten gibbus, Chione cancellata, Mercenaria campechiensis, and Rangia flexuosa). -

0078B-Observacionessobrelamorfo
OBSERVACIONES SOBRE LA MORFOLOGIA VALVAR DE PODODESMUS RUD/5 BRODERIP CON REFERENCIA A LA DIAGNOSIS DE PODODESMUS LELOIRI CARCELLES (BIVALVIA, ANOMllDAE). CIOCCO,Néstorf. * · DIAZ, Miguel Á. ** i RESUMEN · En el golfo San José (Chubut, Argentina, 42° Lat. S.) se encuentran individuos libres y fijos del géne ro Pododesmus cuya morfología coincide con la descripción de P. rudis y P. /e/oiri, respectivamente. Se compara la morfología valvar de ainbos tipos de vida y se estudia la influencia del ambiente sobre las mismas. Se analizan las diferencias entre ambas especies, concluyendo que P. /e/oiri es sólo una forma de P. rudis. SUMMARY Observations on the morphology of Pododesmus rudis with reference.to the diagnosis of Pododesmus /eloiri <:arcelles (Bivalvia, Anomiidae) . · Free · arid attached individuals of Pododesmus were found in San José gulf (Chubut, Argentina, 42 º Lat. S.); according to their morphology, both forms can be assigned to P.rudis and P. /e/oiri, · respectively. The shell morphology of free and attached specimens is compared and the influence of environmental factors on shell shape of both habits is analized. Differences between both species are discussed, concluding thatP. le/oiri is a form of P. rudis. INTRODUCCION . Carcelles (1914, 1944), Castellanos (1967) y Scarabino (1977), citan dos especies del género . Pododesmuspara aguas del Mar Argentino: P. rudis (Broderip, 1834} y P. le/oiriCarcelles, 1941. Ríos (1970-1975} señala la posibilidad de que ésta última sea solo una forma de P. rudis. " Becario de Perfeccionamiento. (CONICET) "* Técnico Prinéipal (CONICET) . Centro Nacional Patagónico (CONlCET) 28 de.Julio Nro. 28 • (9120) Puérto Madryn • Chubut ·Argentina. -

Oyster Research and Restoration in U.S
ACKNOWLEDGEMENTS This work is a result of research sponsored in part by NOAA Office of Sea Grant, U.S. Department of Commerce. The U.S. Government is authorized to produce and distribute reprints for governmental purposes notwithstanding any copyright notation that may appear hereon. VSG-03-03 http://www. virginia.edu/virginia-sea-grant MSG-UM-SG-TS-2003-01 http://www.mdsg.umd.edu OYSTER RESEARCH AND RESTORATION IN U.S. COASTAL WATERS WORKSHOP PURPOSE AND GOALS The NOAA National Sea Grant College program has made a substantial commitment to research on numerous aspects of the domestic oyster fishery. 1bis commitment represents a long-term, focused effort that has led to greater understanding of the biological, molecular genetic and ecological characteristics of these bivalves and the diseases that now impact them in U.S. coastal waters. The achievements of the Oyster Disease Research Program (ODRP) cover multiple areas, including the development of new tools for disease diagnosis, the successful breeding of disease resistant oyster strains, the development of new models of the interaction of disease and environmental factors and the development of a new understanding of the disease process at the cellular level. The Gulf Oyster Industry Program (GOIP) has extended this research to the Gulf of Mexico fishery and in addition has supported innovative research focused on numerous aspects of pathogens in oysters-including rapid detection and enumeration, new processing methods to ensure public health - while gaining an understanding of the impact ofharmful algal species. A strong, collaborative interaction with industry partners has been a hallmark of this program. -

TREATISE ONLINE Number 48
TREATISE ONLINE Number 48 Part N, Revised, Volume 1, Chapter 31: Illustrated Glossary of the Bivalvia Joseph G. Carter, Peter J. Harries, Nikolaus Malchus, André F. Sartori, Laurie C. Anderson, Rüdiger Bieler, Arthur E. Bogan, Eugene V. Coan, John C. W. Cope, Simon M. Cragg, José R. García-March, Jørgen Hylleberg, Patricia Kelley, Karl Kleemann, Jiří Kříž, Christopher McRoberts, Paula M. Mikkelsen, John Pojeta, Jr., Peter W. Skelton, Ilya Tëmkin, Thomas Yancey, and Alexandra Zieritz 2012 Lawrence, Kansas, USA ISSN 2153-4012 (online) paleo.ku.edu/treatiseonline PART N, REVISED, VOLUME 1, CHAPTER 31: ILLUSTRATED GLOSSARY OF THE BIVALVIA JOSEPH G. CARTER,1 PETER J. HARRIES,2 NIKOLAUS MALCHUS,3 ANDRÉ F. SARTORI,4 LAURIE C. ANDERSON,5 RÜDIGER BIELER,6 ARTHUR E. BOGAN,7 EUGENE V. COAN,8 JOHN C. W. COPE,9 SIMON M. CRAgg,10 JOSÉ R. GARCÍA-MARCH,11 JØRGEN HYLLEBERG,12 PATRICIA KELLEY,13 KARL KLEEMAnn,14 JIřÍ KřÍž,15 CHRISTOPHER MCROBERTS,16 PAULA M. MIKKELSEN,17 JOHN POJETA, JR.,18 PETER W. SKELTON,19 ILYA TËMKIN,20 THOMAS YAncEY,21 and ALEXANDRA ZIERITZ22 [1University of North Carolina, Chapel Hill, USA, [email protected]; 2University of South Florida, Tampa, USA, [email protected], [email protected]; 3Institut Català de Paleontologia (ICP), Catalunya, Spain, [email protected], [email protected]; 4Field Museum of Natural History, Chicago, USA, [email protected]; 5South Dakota School of Mines and Technology, Rapid City, [email protected]; 6Field Museum of Natural History, Chicago, USA, [email protected]; 7North -

Studies on Molluscan Shells: Contributions from Microscopic and Analytical Methods
Micron 40 (2009) 669–690 Contents lists available at ScienceDirect Micron journal homepage: www.elsevier.com/locate/micron Review Studies on molluscan shells: Contributions from microscopic and analytical methods Silvia Maria de Paula, Marina Silveira * Instituto de Fı´sica, Universidade de Sa˜o Paulo, 05508-090 Sa˜o Paulo, SP, Brazil ARTICLE INFO ABSTRACT Article history: Molluscan shells have always attracted the interest of researchers, from biologists to physicists, from Received 25 April 2007 paleontologists to materials scientists. Much information is available at present, on the elaborate Received in revised form 7 May 2009 architecture of the shell, regarding the various Mollusc classes. The crystallographic characterization of Accepted 10 May 2009 the different shell layers, as well as their physical and chemical properties have been the subject of several investigations. In addition, many researches have addressed the characterization of the biological Keywords: component of the shell and the role it plays in the hard exoskeleton assembly, that is, the Mollusca biomineralization process. All these topics have seen great advances in the last two or three decades, Shell microstructures expanding our knowledge on the shell properties, in terms of structure, functions and composition. This Electron microscopy Infrared spectroscopy involved the use of a range of specialized and modern techniques, integrating microscopic methods with X-ray diffraction biochemistry, molecular biology procedures and spectroscopy. However, the factors governing synthesis Electron diffraction of a specific crystalline carbonate phase in any particular layer of the shell and the interplay between organic and inorganic components during the biomineral assembly are still not widely known. This present survey deals with microstructural aspects of molluscan shells, as disclosed through use of scanning electron microscopy and related analytical methods (microanalysis, X-ray diffraction, electron diffraction and infrared spectroscopy). -

Diseases of Opakapaka
Opakapaka Diseases of Opakapaka Hawai'i Institute of Marine Biology Michael L. Kent1, Jerry R. Heidel2, Amarisa Marie3, Aaron Moriwake4, Virginia Moriwake4, Benjamin Alexander4, Virginia Watral1, Christopher D. Kelley5 1Center for Fish Disease Research, http://www.oregonstate.edu/dept/salmon/, Department of Microbiology, 220 Nash Hall, Oregon State University, Corvallis, OR 97331 2Director, Veterinary Diagnostic Laboratory, College of Veterinary Medicine, Oregon State University Corvallis, OR 97331-3804 3Department of Fisheries and Wildlife, Oregon State University, Corvallis, OR 97331 4Hawaii Institute of Marine Biology, http://www.hawaii.edu/HIMB/, 46-007 Lilipuna Road, Kaneohe, HI 96744 5Hawaii Undersea Research Laboratory University of Hawaii, 1000 Pope Rd, MSB 303 Honolulu, HI 96744 Introduction Opakapaka (Pristipomoides filamentosus), a highly valued commercial deep-water snapper is under investigation at the University of Hawaii through support of Department of Land and Natural Resources. The purpose of this endeavor is to develop aquaculture methods for enhancement of wild stocks of this fish and related species (e.g., ehu, onaga) as well as development of this species of commercial aquaculture. The usual scenario for investigations or identification of serious diseases in aquaculture is that as the culture of a fish species expands, devastating economic and biological losses due to "new" or "unknown" diseases follow, and then research is conducted to identify their cause and develop methods for their control. In contrast, the purpose of this study was to provide a proactive approach to identify potential health problems that may be encountered in opakapaka before large-scale culture of this species is underway. A few diseases have already been recognized as potential problems for the culture of captive opakapaka. -

Full Text in Pdf Format
MARINE ECOLOGY PROGRESS SERIES Vol. 118: 167-177, 1995 Published March 9 Mar. Ecol. Prog. Ser. Prey preferences of blue crabs Callinectes sapidus feeding on three bivalve species Elizabeth L. Ebersole*, Victor S. Kennedy*' University of Maryland System, Horn Point Environmental Laboratory, PO Box 775, Cambridge. Maryland 21613. USA ABSTRACT: Individual blue crabs Callinectes sapidus were allowed to forage on 3 bivalve species (soft clam Mya arenaria; Atlantic rangia clam Rangia cuneata; hooked mussel Ischadium recurvum), with 2 of the 3 species made available together at one time in 220 1 aquaria. In 3 separate sets of experiments, we examined the blue crab's consumption and preferences between 2 bivalve species of different prof- itabilities [(netenergy intake)/(handling time); J S-']: M. arenaria and R. cuneata, M. arenaria and I. recurvum, and R. cuneata and I. recurvum. These experiments also examined the effects of 3 additional factors on prey consumption and prey preference: prey location (near to or distant from point of intro- duction of crab), prey refuge availability (shallow or deep sand for the clams; detached or clustered for the hooked mussel), and prey density (high or low numbers). Profitability curves correctly predicted that the blue crab preferred the highly profitable soft clam over the less energetically profitable Atlantic rangm clam. When the difference between prey profitabllities was not as great (i.e. between the soft dam and the hooked mussel, and between the Atlantic rangia clam and the hooked mussel) profitability alone was not a clear predictor of blue crab preference. Prey refuge availability signifi- cantly affected prey preference; deep sand provided (1)a greater refuge for the soft clam than for the Atlantic rangia clam and (2) a greater refuge for the soft clam than clustering provided for the hooked mussel. -

D070p001.Pdf
DISEASES OF AQUATIC ORGANISMS Vol. 70: 1–36, 2006 Published June 12 Dis Aquat Org OPENPEN ACCESSCCESS FEATURE ARTICLE: REVIEW Guide to the identification of fish protozoan and metazoan parasites in stained tissue sections D. W. Bruno1,*, B. Nowak2, D. G. Elliott3 1FRS Marine Laboratory, PO Box 101, 375 Victoria Road, Aberdeen AB11 9DB, UK 2School of Aquaculture, Tasmanian Aquaculture and Fisheries Institute, CRC Aquafin, University of Tasmania, Locked Bag 1370, Launceston, Tasmania 7250, Australia 3Western Fisheries Research Center, US Geological Survey/Biological Resources Discipline, 6505 N.E. 65th Street, Seattle, Washington 98115, USA ABSTRACT: The identification of protozoan and metazoan parasites is traditionally carried out using a series of classical keys based upon the morphology of the whole organism. However, in stained tis- sue sections prepared for light microscopy, taxonomic features will be missing, thus making parasite identification difficult. This work highlights the characteristic features of representative parasites in tissue sections to aid identification. The parasite examples discussed are derived from species af- fecting finfish, and predominantly include parasites associated with disease or those commonly observed as incidental findings in disease diagnostic cases. Emphasis is on protozoan and small metazoan parasites (such as Myxosporidia) because these are the organisms most likely to be missed or mis-diagnosed during gross examination. Figures are presented in colour to assist biologists and veterinarians who are required to assess host/parasite interactions by light microscopy. KEY WORDS: Identification · Light microscopy · Metazoa · Protozoa · Staining · Tissue sections Resale or republication not permitted without written consent of the publisher INTRODUCTION identifying the type of epithelial cells that compose the intestine.