Morphotypic Diversity of Microalgae from Arid Zones of Rajasthan (India)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rajasthan Urban Sector Development Investment Program - Nagaur Water Supply
Initial Environmental Examination Document Stage: Revised Project Number: 40031-053 February 2014 IND: Rajasthan Urban Sector Development Investment Program - Nagaur Water Supply Subproject (Additional Scope) The work of water supply subproject is already under execution in Tr-02. The scope of works under execution includes construction/rehabilitation of distribution network construction of raising mains, OHSRs, CWR and an intermediate pump house. Some additional areas are proposed to lay distribution network and construction of ESRs. Prepared by Local Self Government Department For the Government of Rajasthan Rajasthan Urban Infrastructure Development Project The initial environmental examination is a document of the borrower. The views expressed herein do not necessarily represent those of ADB’s Board of Directors, Management, or staff, and may be preliminary in nature. ABBREVIATION ADB - Asian Development Bank DSC - Design and Supervision Consultancy EA - Executing Agency EAC - Expert Appraisal Committee FI - Financial Intermediary GLSR - Ground Level Service Reservoir GoI - Government of India GoR - Government of Rajasthan GSI - Geological Survey of India IA - Implementing Agency IEE - Initial Environmental Examination IPMC - Investment Programme Management Consultancy IPMU - Investment Programme Management Unit JNNURM - Jawaharlal Nehru National Urban Renewal Mission LSGD - Local Self-Government Department MFF - Multitranche Financing Facility MoEF - Ministry of Environment and Forests NAAQS - National Ambient Air Quality Standards -

Natural Product Gene Clusters in the Filamentous Nostocales Cyanobacterium HT-58-2
life Article Natural Product Gene Clusters in the Filamentous Nostocales Cyanobacterium HT-58-2 Xiaohe Jin 1,*, Eric S. Miller 2 and Jonathan S. Lindsey 1 1 Department of Chemistry, North Carolina State University, Raleigh, NC 27695-8204, USA; [email protected] 2 Department of Plant and Microbial Biology, North Carolina State University, Raleigh, NC 27695-7615, USA; [email protected] * Correspondence: [email protected] Abstract: Cyanobacteria are known as rich repositories of natural products. One cyanobacterial- microbial consortium (isolate HT-58-2) is known to produce two fundamentally new classes of natural products: the tetrapyrrole pigments tolyporphins A–R, and the diterpenoid compounds tolypodiol, 6-deoxytolypodiol, and 11-hydroxytolypodiol. The genome (7.85 Mbp) of the Nostocales cyanobacterium HT-58-2 was annotated previously for tetrapyrrole biosynthesis genes, which led to the identification of a putative biosynthetic gene cluster (BGC) for tolyporphins. Here, bioinformatics tools have been employed to annotate the genome more broadly in an effort to identify pathways for the biosynthesis of tolypodiols as well as other natural products. A putative BGC (15 genes) for tolypodiols has been identified. Four BGCs have been identified for the biosynthesis of other natural products. Two BGCs related to nitrogen fixation may be relevant, given the association of nitrogen stress with production of tolyporphins. The results point to the rich biosynthetic capacity of the HT-58-2 cyanobacterium beyond the production of tolyporphins and tolypodiols. Citation: Jin, X.; Miller, E.S.; Lindsey, J.S. Natural Product Gene Clusters in Keywords: anatoxin-a/homoanatoxin-a; hapalosin; heterocyst glycolipids; natural products; sec- the Filamentous Nostocales ondary metabolites; shinorine; tolypodiols; tolyporphins Cyanobacterium HT-58-2. -

Anabaena Variabilis ATCC 29413
Standards in Genomic Sciences (2014) 9:562-573 DOI:10.4056/sig s.3899418 Complete genome sequence of Anabaena variabilis ATCC 29413 Teresa Thiel1*, Brenda S. Pratte1 and Jinshun Zhong1, Lynne Goodwin 5, Alex Copeland2,4, Susan Lucas 3, Cliff Han 5, Sam Pitluck2,4, Miriam L. Land 6, Nikos C Kyrpides 2,4, Tanja Woyke 2,4 1Department of Biology, University of Missouri-St. Louis, St. Louis, MO 2DOE Joint Genome Institute, Walnut Creek, CA 3Lawrence Livermore National Laboratory, Livermore, CA 4Lawrence Berkeley National Laboratory, Berkeley, CA 5Los Alamos National Laboratory, Los Alamos, NM 6Oak Ridge National Laboratory, Oak Ridge, TN *Correspondence: Teresa Thiel ([email protected]) Anabaena variabilis ATCC 29413 is a filamentous, heterocyst-forming cyanobacterium that has served as a model organism, with an extensive literature extending over 40 years. The strain has three distinct nitrogenases that function under different environmental conditions and is capable of photoautotrophic growth in the light and true heterotrophic growth in the dark using fructose as both carbon and energy source. While this strain was first isolated in 1964 in Mississippi and named Ana- baena flos-aquae MSU A-37, it clusters phylogenetically with cyanobacteria of the genus Nostoc . The strain is a moderate thermophile, growing well at approximately 40° C. Here we provide some additional characteristics of the strain, and an analysis of the complete genome sequence. Introduction Classification and features Anabaena variabilis ATCC 29413 (=IUCC 1444 = The general characteristics of A. variabilis are PCC 7937) is a semi-thermophilic, filamentous, summarized in Table 1 and its phylogeny is shown heterocyst-forming cyanobacterium. -
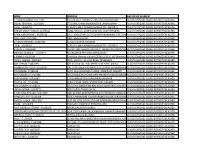
Name Address Nature of Payment P
NAME ADDRESS NATURE OF PAYMENT P. NAVEENKUMAR -91774443 NO 139 KALATHUMEDU STREETMELMANAVOOR0 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED VISHAL TEKRIWAL -31262196 27,GOPAL CHANDRAMUKHERJEE LANEHOWRAH CLAIMS CHEQUES ISSUED BUT NOT ENCASHED LOCAL -16280591 #196 5TH MAIN ROADCHAMRAJPETPH 26679019 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED BHIKAM SINGH THAKUR -21445522 JABALPURS/O UDADET SINGHVILL MODH PIPARIYA CLAIMS CHEQUES ISSUED BUT NOT ENCASHED ATINAINARLINGAM S -91828130 NO 2 HINDUSTAN LIVER COLONYTHAGARAJAN STREET PAMMAL0CLAIMS CHEQUES ISSUED BUT NOT ENCASHED USHA DEVI -27227284 VPO - SILOKHARA00 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED SUSHMA BHENGRA -19404716 A-3/221,SECTOR-23ROHINI CLAIMS CHEQUES ISSUED BUT NOT ENCASHED LOCAL -16280591 #196 5TH MAIN ROADCHAMRAJPETPH 26679019 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED RAKESH V -91920908 NO 304 2ND FLOOR,THIRUMALA HOMES 3RD CROSS NGRLAYOUT,CLAIMS CHEQUES ROOPENA ISSUED AGRAHARA, BUT NOT ENCASHED KRISHAN AGARWAL -21454923 R/O RAJAPUR TEH MAUCHITRAKOOT0 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED K KUMAR -91623280 2 nd floor.olympic colonyPLOT NO.10,FLAT NO.28annanagarCLAIMS west, CHEQUES ISSUED BUT NOT ENCASHED MOHD. ARMAN -19381845 1571, GALI NO.-39,JOOR BAGH,TRI NAGAR0 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED ANIL VERMA -21442459 S/O MUNNA LAL JIVILL&POST-KOTHRITEH-ASHTA CLAIMS CHEQUES ISSUED BUT NOT ENCASHED RAMBHAVAN YADAV -21458700 S/O SURAJ DEEN YADAVR/O VILG GANDHI GANJKARUI CHITRAKOOTCLAIMS CHEQUES ISSUED BUT NOT ENCASHED MD SHADAB -27188338 H.NO-10/242 DAKSHIN PURIDR. AMBEDKAR NAGAR0 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED MD FAROOQUE -31277841 3/H/20 RAJA DINENDRA STREETWARD NO-28,K.M.CNARKELDANGACLAIMS CHEQUES ISSUED BUT NOT ENCASHED RAJIV KUMAR -13595687 CONSUMER APPEALCONSUMERCONSUMER CLAIMS CHEQUES ISSUED BUT NOT ENCASHED MUNNA LAL -27161686 H NO 524036 YARDS, SECTOR 3BALLABGARH CLAIMS CHEQUES ISSUED BUT NOT ENCASHED SUNIL KUMAR -27220272 S/o GIRRAJ SINGHH.NO-881, RAJIV COLONYBALLABGARH CLAIMS CHEQUES ISSUED BUT NOT ENCASHED DIKSHA ARORA -19260773 605CELLENO TOWERDLF IV CLAIMS CHEQUES ISSUED BUT NOT ENCASHED R. -

Nagaur District Survey Report
NAGAUR DISTRICT SURVEY REPORT 1 CONTENTS PAGE(S) 1. INTRODUCTION 2 2. OVERVIEW OF MINING ACTIVITY IN THE DISTRICT 8 3. THE LIST OF MINING LEASES IN THE DISTRICT 39 4. DETAILS OF ROYALTY OR REVENUE RECEIVED IN LAST 147 THREE YEARS 5. DETAILS OF PRODUCTION OF SAND OR BAJARI OR 150 MINOR MINERALS IN LAST THREE YEARS 6. PROCESS OF DEPOSITION OF SEDIMENTS IN THE RIVERS OF 152 THE DISTRICT 7. GENERAL PROFILE OF THE DISTRICT 152 8. LAND UTILIZATION PATTERN IN THE DISTRICT 161 9. PHYSIOGRAHY OF THE DISTRICT 163 10. RAINFALL 165 11. GEOLOGY AND MINERAL WEALTH 166 2 [ Nagaur District Survey Report CHAPTER 1: Introduction Nagaur district falls almost in the central part of Rajasthan covering an area of 17,718 sq.km. The district is bounded by the latitudes 26°02'12" to 27°37'39" and longitudes 73°05'20" to 75°24'. The NH-65 which connects district H.Q. with Jodhpur and NH-89 connecting it with Ajmer and Bikaner are passing through the district. It is also connected with Jaipur, Jodhpur and Bikaner through broad gauge railway line. It is oval in shape. The district forms a part of great Thar desert and a large part of it is covered by wind blown sand. The district boundary is shared by seven districts of Rajasthan viz.-Jaipur, Ajmer, Pali, Jodhpur, Bikaner, Churu and Sikar. It falls in Ajmer division and administratively divided into four sub divisional offices viz. Didwana, Nagaur, Merta, and Parbatsar. Nagaur, Merta, Jayal, Ladnun, Didwana, Nawa, Makrana, Degana, Parbatsar and Khimsar are the ten tehsil head quaters of the district. -

Local Hopping Mobile DNA Implicated in Pseudogene Formation And
Vigil-Stenman et al. BMC Genomics (2015) 16:193 DOI 10.1186/s12864-015-1386-7 RESEARCH ARTICLE Open Access Local hopping mobile DNA implicated in pseudogene formation and reductive evolution in an obligate cyanobacteria-plant symbiosis Theoden Vigil-Stenman1*, John Larsson2, Johan A A Nylander3 and Birgitta Bergman1 Abstract Background: Insertion sequences (ISs) are approximately 1 kbp long “jumping” genes found in prokaryotes. ISs encode the protein Transposase, which facilitates the excision and reinsertion of ISs in genomes, making these sequences a type of class I (“cut-and-paste”) Mobile Genetic Elements. ISs are proposed to be involved in the reductive evolution of symbiotic prokaryotes. Our previous sequencing of the genome of the cyanobacterium ‘Nostoc azollae’ 0708, living in a tight perpetual symbiotic association with a plant (the water fern Azolla), revealed the presence of an eroding genome, with a high number of insertion sequences (ISs) together with an unprecedented large proportion of pseudogenes. To investigate the role of ISs in the reductive evolution of ‘Nostoc azollae’ 0708, and potentially in the formation of pseudogenes, a bioinformatic investigation of the IS identities and positions in 47 cyanobacterial genomes was conducted. To widen the scope, the IS contents were analysed qualitatively and quantitatively in 20 other genomes representing both free-living and symbiotic bacteria. Results: Insertion Sequences were not randomly distributed in the bacterial genomes and were found to transpose short distances from their original location (“local hopping”) and pseudogenes were enriched in the vicinity of IS elements. In general, symbiotic organisms showed higher densities of IS elements and pseudogenes than non-symbiotic bacteria. -
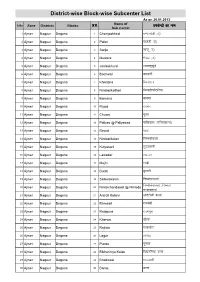
SC Nagaur.Pdf
District-wise Block-wise Subcenter List As on 26.01.2013 Name of S.No Zone Districts Blocks Ø-la- Sub Center midsUnzks dk uke 1 Ajmer Nagaur Degana 1 Champakhedi pEik[ksMh (m½ 2 Ajmer Nagaur Degana 2 Palari ikyMh (m½ 3 Ajmer Nagaur Degana 3 Sanju lkatw (m½ 4 Ajmer Nagaur Degana 4 Mewara esoMk (m½ 5 Ajmer Nagaur Degana 5 Jaalsukhurd tkylw[kqnZ 6 Ajmer Nagaur Degana 6 Bachwari cNokjh 7 Ajmer Nagaur Degana 7 Khiwtana f[koarkuk 8 Ajmer Nagaur Degana 8 Nimbarikothari fuEcMhdksBfj;k 9 Ajmer Nagaur Degana 9 Bamana cke.kk 10 Ajmer Nagaur Degana 10 Rajod jktksn 11 Ajmer Nagaur Degana 11 Chuwa pqvk 12 Ajmer Nagaur Degana 12 Paliyas @ Paliyawas ikfy;kl (ikfy;kokl½ 13 Ajmer Nagaur Degana 13 Rewat jsoar 14 Ajmer Nagaur Degana 14 Nimbarikalan fuEcMhdyka 15 Ajmer Nagaur Degana 15 Kutyasani dqR;kluh 16 Ajmer Nagaur Degana 16 Lawadar yoknj 17 Ajmer Nagaur Degana 17 Majhi eka>h 18 Ajmer Nagaur Degana 18 Butati cqVkVh 19 Ajmer Nagaur Degana 19 Sinbolakalan fuEcksykdyka fuEchpankork (fuEcMk 20 Ajmer Nagaur Degana 20 Nimbichandawat @ Nimbda Candawtan pUnkorku½ 21 Ajmer Nagaur Degana 21 Antroli Kalann vkarjksyh dyka 22 Ajmer Nagaur Degana 22 Ramsari jkeljh 23 Ajmer Nagaur Degana 23 Rajapura jktkiqjk 24 Ajmer Nagaur Degana 24 Kherwa [kSjok 25 Ajmer Nagaur Degana 25 Rajlota jktyksrk 26 Ajmer Nagaur Degana 26 Lagor yaxksM 27 Ajmer Nagaur Degana 27 Punas iwukl 28 Ajmer Nagaur Degana 28 Bikhariniya Kalan fc[kjfu;k dyka 29 Ajmer Nagaur Degana 29 Bhadwasi Hkknoklh 30 Ajmer Nagaur Degana 30 Barna cjuk Name of S.No Zone Districts Blocks Ø-la- Sub Center midsUnzks dk -

Degana Tungsten Project—Present Plant
Bull. Mater. Sci., Vol. 19, No. 2, April 1996, pp. 313 - 329. ~ Printed in India. Degana tungsten project--present plant practice and future scenario M R JAKHU and S RAY Hindustan Zinc Ltd, Yashad Bhavan, Udaipur 313 001, India Abstract. Experiments/testworks were carried out on Degana tungsten ore by various R & D organizations to recover the strategic mineral wolframite. Process flowsheet developed after tests on the dump ore assaying 0.151% WO3 is being tried on a very small scale till the 150 tpd pilot beneficiation plant is commissioned. Tungsten bearing granite samples were also found amenable to physical beneficiation. Hence 168 Mt granitic resources analysing 0.08% WO 3 has ushered a hope for large scale exploitation subject to detailed exploration. By-product recovery of lithium, caesium, rubidium and some other trace elements, if feasible, will be of added advantage. Keywords. Degana tungsten; present practice; future scenario. 1. Introduction Degana Tungsten Project in Nagaur District (Rajasthan) is located midway between Jaipur and Jodhpur, and about 82 km from Ajmer by road. Since discovery in 1912, it was sporadically exploited by various agencies with peak operation during the world wars. The Directorate of Mines and Geology (DMG), Rajasthan, started its operation after the Indo-China war in 1962. The operation continued subsequently by Rajasthan State Industrial and Mining Development Corporation (RSIMDC), Rajasthan State Mineral Development Corporation Ltd. (RSMDC) and Rajasthan State Tungsten Development Corporation Ltd. (RSTDC) by selective underground vein mining with concentration by manual sorting and panning. Due to low tenor and limited reserve, production from underground operation was limited and the beneficiation process was made exclusively manual. -

KNIGHT-DISSERTATION-2014.Pdf
Copyright by Rebecca Anne Knight 2014 The Dissertation Committee for Rebecca Anne Knight Certifies that this is the approved version of the following dissertation: Coordinated Response and Regulation of Carotenogenesis in Thermosynechococcus elongatus (BP-1): Implications for Commercial Application Committee: Jerry J. Brand, Supervisor Hal S. Alper, Co-Supervisor Enamul Huq Robert K. Jansen David R. Nobles Coordinated Response and Regulation of Carotenogenesis in Thermosynechococcus elongatus (BP-1): Implications for Commercial Application by Rebecca Anne Knight, B.A., B.S.E., M.S.E. Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin December 2014 Dedication Dedicated to my parents, who taught me to always work hard, and never stop learning. Acknowledgements I think there is a unique experience that people like myself undergo when working in industry then returning to academia. Very little is taken for granted on campus, so much to learn and take advantage of knowledge-wise. Then there is the flip side; long hours in the lab, the struggle to find your niche and contribution to the scientific community, and a much tighter budget both for research and personal life. I am here to say that without my family and their encouragement and sacrifices I would not have made it. My loving husband, who’s been my biggest supporter, and my wonderful step-daughters, whom I have dragged to campus numerous times and have essentially lived in my shoes for six whole years. -

Ladnu Sewerage Subproject, District-Nagaur, Rajasthan
Initial Environmental Examination Document Stage: Draft Project Number: 42267-031 May 2020 IND: Rajasthan Secondary Towns Development Sector Project – Ladnu Sewerage Subproject, District-Nagaur, Rajasthan Prepared by Project Management Unit, Rajasthan Urban Drinking Water Sewerage and Infrastructure Corporation Limited, Government of Rajasthan for the Asian Development Bank. This initial environmental examination is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, management, or staff, and may be preliminary in nature. Your attention is directed to the “terms of use” section of this website. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. CURRENCY EQUIVALENTS (As of 29 May 2020) Currency unit – Indian rupee (₹) ₹1.00 = $ 0.01321 $1.00 = ₹ 75.7085 ABBREVIATIONS ACM – Asbestos containing Material ADB – Asian Development Bank BOCW – Building and Other Construction Workers CGWB – Central Ground Water Board CLC – City Level Committee CPCB – Central Pollution Control Board CPHEEO – Central Public Health and Environmental Engineering Organization CTE – consent to establish CTO – consent to operate CWR – Clear Water Reservoir DBO – Design-Build-Operate DPR – Detailed Project Report EHS – Environmental Health and Safety EIA – Environmental -
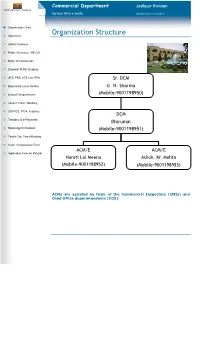
Organization Structure
Commercial Department Jodhpur Division North Western Railway Service With a Smile. (Updated Upto 31.08.2011) Organization Chart Objectives Organization Structure Salient Features Public Grievance /NR Cell Right To Information Coolies/ R.Rs/ Enquiry UTS, PRS, UTS Cum PRS Sr. DCM Registered Lease Holders G. N. Sharma Leased Compartments (Mobile-9001198950) Comml. Plots/ Handling STD/PCO, RTSA, Pay&Use DCM Tenders & e-Payment Dhirumal Stacking Permission (Mobile-9001198951) Tourist Car, Coach Booking Claim Compensation Form ACM/E ACM/E Application Form for Refund Hareti Lal Meena Ashok. Kr. Mehta (Mobile-9001198952) (Mobile-9001198953) ACMs are assisted by team of the Commercial Inspectors (CMIs) and Chief Office Superintendents (COS). Commercial Department Jodhpur Division Service With a Smile. (Updated Upto 31.08.2011) Organization Chart Objectives Objectives of Commercial Department Salient Features The commercial Department is the interface between the Public Grievance /NR Cell Railways and its customers, ensuring comfortable, safe and Right To Information secure journey to its passengers. On Freight side it is looking after marketing and transportation of Goods. Coolies/ R.Rs/ Enquiry It is also taking care of passenger amenities at the stations and UTS, PRS, UTS Cum PRS maintaining friendly relation with the passengers and traders. Registered Lease Holders The fixing of rates, fares and other charges and the correct collection, accountal and remittance of traffic receipts are also Leased Compartments among its functions. Comml. Plots/ Handling STD/PCO, RTSA, Pay&Use Tenders & e-Payment Stacking Permission Tourist Car, Coach Booking Claim Compensation Form Application Form for Refund Commercial Department Jodhpur Division North Western Railway Service With a Smile. -

1497857537315-Map.Pdf
NORTH WESTERN RAILWAY SYSTEMAS ON MAP 17-06-2017 SANCTIONED WORKS (IN PROGRESS NOT COMMISSIONED) BATHINDA LIST OF SANCTIONED WORKS OVER NWR SALIENT FEATURES PHYSICAL PUNJAB Sr. PROGRESS MANDIDABWALI No. NAME OF WORK YEAR OF COST LENGTH SANCTION IN CR IN KMS & TDC SRIGANGANAGER KALANWALI I.0 NEW LINE (07) HARYANA 1.1 DAUSA(DO) -GANGAPUR CITY(GGC) 1996-97 780.98 92.67 53% SIRSA (i)Revised Estimate of Rs780.98cr sanction by Rly Bd.on 07.03.17. 1.DO-Deedwana, June 17 GAJSINGHPUR 2.GGC-Khuntla, Feb 18 PELIBANGA HANUMANGARH 1.2 RATLAM(RTM)-DUNGARPUR(DNRP)-Via-BANSWARA(BNSW) 2011-12 2417.24188.85 6.25% TO DHURI (i)Rly. Bd. vide letter dt. 30.03.17 directed to freeze all works. Not fixed SARUPSAR 1.3 THIAYAT HAMIRA(THM)-SANU 2013-14 262.46 58.00 25% SURATGARH (i)Land aquisition for forest & private land is still in progress. Jan 18 ANUPGARH (ii)NOC for construction of ROB is still awaited from State Govt PIPREN ROHTAK 1.4 AJMER(AII)-KOTA(KTT)(NASEERABAD(NSD)-JALINDRI) 2013-14 822.00 145.00 Not fixed BIRADWAL NOHAR (i)CCEA sanction is yet to be obtained by Rly Bd. HISAR 1.5 PUSHKAR(POHT)- MERTA(MTD) 2013-14 323.00 59.00 Not fixed PAKISTAN (i)CCEA sanction is yet to be obtained by Rly Bd. 1.6 AJMER(NASIRABAD)-SAWAI MADHOPUR(SWM) 2015-16 873.71 165.00 Not fixed (CHAUTH KA BARWARA) (CKB)Via TONK (i)Chief Secy./Govt.of Raj. vide D.O.letter no.F-26(7)-para/RC/2013/25409 LUNKARAN SURATPURA SAR BHIWANI Dt.09.12.2016 reqested railways to share full cost.of sanctioned works in progress SADULPUR NEW DELHI & others future projects.