Mapping Nanoscale Electromagnetic Near-Field Distributions Using Optical Forces
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Engineering Electromagnetic Wave Properties Using Subwavelength Antennas Structures
ENGINEERING ELECTROMAGNETIC WAVE PROPERTIES USING SUBWAVELENGTH ANTENNAS STRUCTURES Dissertation Submitted to The School of Engineering of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree Doctor of Philosophy in Electro-Optics By Shiyi Wang UNIVERSITY OF DAYTON Dayton, Ohio May, 2015 ENGINEERING ELECTROMAGNETIC WAVE PROPERTIES USING SUBWAVELENGTH ANTENNAS STRUCTURES Name: Wang, Shiyi APPROVED BY: ____________________________ ____________________________ Qiwen Zhan, Ph.D. Partha Banerjee, Ph.D. Advisory Committee Chairman Committee Member Professor Director and Professor Electro-Optics Program Electro-Optics Program ____________________________ ____________________________ Andrew Sarangan, Ph.D. Imad Agha, Ph.D. Committee Member Committee Member Professor Assistant Professor Electro-Optics Program Physics ____________________________ ____________________________ John G. Weber, Ph.D. Eddy M. Rojas, Ph.D., M.A., P.E. Associate Dean Dean, School of Engineering School of Engineering ii © Copyright by Shiyi Wang All rights reserved 2015 iii ABSTRACT ENGINEERING ELECTROMAGNETIC WAVE PROPERTIES USING SUBWAVELENGTH ANTENNAS STRUCTURES Name: Wang, Shiyi University of Dayton Advisor: Dr. Qiwen Zhan With extraordinary properties, generation of complex electromagnetic field based on novel subwavelength antennas structures has attracted great attentions in many areas of modern nano science and technology, such as compact RF sensors, micro-wave receivers and nano- antenna-based optical/IR devices. This dissertation -

SOLID STATE PHYSICS PART II Optical Properties of Solids
SOLID STATE PHYSICS PART II Optical Properties of Solids M. S. Dresselhaus 1 Contents 1 Review of Fundamental Relations for Optical Phenomena 1 1.1 Introductory Remarks on Optical Probes . 1 1.2 The Complex dielectric function and the complex optical conductivity . 2 1.3 Relation of Complex Dielectric Function to Observables . 4 1.4 Units for Frequency Measurements . 7 2 Drude Theory{Free Carrier Contribution to the Optical Properties 8 2.1 The Free Carrier Contribution . 8 2.2 Low Frequency Response: !¿ 1 . 10 ¿ 2.3 High Frequency Response; !¿ 1 . 11 À 2.4 The Plasma Frequency . 11 3 Interband Transitions 15 3.1 The Interband Transition Process . 15 3.1.1 Insulators . 19 3.1.2 Semiconductors . 19 3.1.3 Metals . 19 3.2 Form of the Hamiltonian in an Electromagnetic Field . 20 3.3 Relation between Momentum Matrix Elements and the E®ective Mass . 21 3.4 Spin-Orbit Interaction in Solids . 23 4 The Joint Density of States and Critical Points 27 4.1 The Joint Density of States . 27 4.2 Critical Points . 30 5 Absorption of Light in Solids 36 5.1 The Absorption Coe±cient . 36 5.2 Free Carrier Absorption in Semiconductors . 37 5.3 Free Carrier Absorption in Metals . 38 5.4 Direct Interband Transitions . 41 5.4.1 Temperature Dependence of Eg . 46 5.4.2 Dependence of Absorption Edge on Fermi Energy . 46 5.4.3 Dependence of Absorption Edge on Applied Electric Field . 47 5.5 Conservation of Crystal Momentum in Direct Optical Transitions . 47 5.6 Indirect Interband Transitions . -

Parametric Amplification of Optical Phonons
Parametric amplification of optical phonons A. Cartellaa,1, T. F. Novaa,b, M. Fechnera, R. Merlinc, and A. Cavalleria,b,d aCondensed Matter Dynamics Department, Max Planck Institute for the Structure and Dynamics of Matter, 22761 Hamburg, Germany; bThe Hamburg Centre for Ultrafast Imaging, University of Hamburg, 22761 Hamburg, Germany; cDepartment of Physics, University of Michigan, Ann Arbor, MI 48109-1040; and dDepartment of Physics, Clarendon Laboratory, University of Oxford, OX1 3PU Oxford, United Kingdom Edited by Peter T. Rakich, Yale University, New Haven, CT, and accepted by Editorial Board Member Anthony Leggett October 22, 2018 (received for review June 6, 2018) We use coherent midinfrared optical pulses to resonantly excite The second contribution to the nonlinear polarization emerges large-amplitude oscillations of the Si–C stretching mode in silicon from the dielectric screening of the electric field E by the elec- carbide. When probing the sample with a second pulse, we ob- trons, giving the term P∞ = e0χE = e0ð«∞ − 1ÞE. In contrast to the serve parametric optical gain at all wavelengths throughout the Born effective charge, which is a pure ionic response, the per- reststrahlen band. This effect reflects the amplification of light by mittivity «∞ accounts for higher-energy excitations of the elec- phonon-mediated four-wave mixing and, by extension, of optical- tronic band structure such as interband transitions. Similar to the phonon fluctuations. Density functional theory calculations clarify Born effective charge, the permittivity «∞ is a constant for small aspects of the microscopic mechanism for this phenomenon. The lattice displacements but becomes dependent on Q when the high-frequency dielectric permittivity and the phonon oscillator lattice is strongly distorted and hence the band structure strength depend quadratically on the lattice coordinate; they os- changes. -

The Nonlinear Optical Susceptibility
Chapter 1 The Nonlinear Optical Susceptibility 1.1. Introduction to Nonlinear Optics Nonlinear optics is the study of phenomena that occur as a consequence of the modification of the optical properties of a material system by the pres- ence of light. Typically, only laser light is sufficiently intense to modify the optical properties of a material system. The beginning of the field of nonlin- ear optics is often taken to be the discovery of second-harmonic generation by Franken et al. (1961), shortly after the demonstration of the first working laser by Maiman in 1960.∗ Nonlinear optical phenomena are “nonlinear” in the sense that they occur when the response of a material system to an ap- plied optical field depends in a nonlinear manner on the strength of the optical field. For example, second-harmonic generation occurs as a result of the part of the atomic response that scales quadratically with the strength of the ap- plied optical field. Consequently, the intensity of the light generated at the second-harmonic frequency tends to increase as the square of the intensity of the applied laser light. In order to describe more precisely what we mean by an optical nonlinear- ity, let us consider how the dipole moment per unit volume, or polarization P(t)˜ , of a material system depends on the strength E(t)˜ of an applied optical ∗ It should be noted, however, that some nonlinear effects were discovered prior to the advent of the laser. The earliest example known to the authors is the observation of saturation effects in the luminescence of dye molecules reported by G.N. -

Optical Properties of Thin-Film High-Temperature Magnetic Ferrites
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2018 Optical Properties of Thin-Film High-Temperature Magnetic Ferrites Brian Scott Holinsworth University of Tennessee, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Recommended Citation Holinsworth, Brian Scott, "Optical Properties of Thin-Film High-Temperature Magnetic Ferrites. " PhD diss., University of Tennessee, 2018. https://trace.tennessee.edu/utk_graddiss/4864 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Brian Scott Holinsworth entitled "Optical Properties of Thin-Film High-Temperature Magnetic Ferrites." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Chemistry. Janice Musfeldt, Major Professor We have read this dissertation and recommend its acceptance: Charles S. Feigerle, Veerle Keppens, Ziling Xue Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Optical Properties of Thin-Film High-Temperature Magnetic Ferrites A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Brian Scott Holinsworth May 2018 Acknowledgments First and foremost I wish to thank my advisor, Professor Janice L. -

Optical-Field-Controlled Photoemission from Plasmonic Nanoparticles
LETTERS PUBLISHED ONLINE: 19 DECEMBER 2016 | DOI: 10.1038/NPHYS3978 Optical-field-controlled photoemission from plasmonic nanoparticles William P. Putnam1,2*, Richard G. Hobbs2,3, Phillip D. Keathley2, Karl K. Berggren2 and Franz X. Kärtner1,2,4 At high intensities, light–matter interactions are controlled by regime. When a nanotip is illuminated by a femtosecond laser pulse, the electric field of the exciting light. For instance, when an the incident field is locally enhanced at the apex of the tip. Due pri- intense laser pulse interacts with an atomic gas, individual marily to the tip's sharp geometry, the field enhancement is typically cycles of the incident electric field ionize gas atoms and steer <10, and the temporal profile of the enhanced field, Ftip.t/, approxi- the resulting attosecond-duration electrical wavepackets1,2. mately follows that of the instantaneous incident field21,22. With typ- Such field-controlled light–matter interactions form the basis ical incident intensities, Ftip.t/ can drive strong-field processes: pho- of attosecond science and have recently expanded from toemission current yields and photoelectron energy spectra from gases to solid-state nanostructures3–18. Here, we extend these nanotips have shown strong-field characteristics3–5,7,10,11,14,15, and field-controlled interactions to metallic nanoparticles support- exciting nanotips with phase-stabilized laser pulses, CEP-sensitive ing localized surface plasmon resonances. We demonstrate signatures have been observed5,14. strong-field, carrier-envelope-phase-sensitive photoemission Compared with nanotips, metallic nanoparticles offer higher from arrays of tailored metallic nanoparticles, and we show field enhancements as well as additional resonant and geometric the influence of the nanoparticle geometry and the plasmon degrees of freedom. -

Lecture Notes on ELECTROMAGNETIC FIELDS AND
Lecture Notes on ELECTROMAGNETIC FIELDS AND WAVES (227-0052-10L) Prof. Dr. Lukas Novotny ETH Z¨urich, Photonics Laboratory February 9, 2013 Introduction The properties of electromagnetic fields and waves are most commonly discussed in terms of the electric field E(r, t) and the magnetic induction field B(r, t). The vector r denotes the location in space where the fields are evaluated. Similarly, t is the time at which the fields are evaluated. Note that the choice of E and B is ar- bitrary and that one could also proceed with combinations of the two, for example, with the vector and scalar potentials A and φ, respectively. The fields E and B have been originally introduced to escape the dilemma of “action-at-distance’, that is, the question of how forces are transferred between two separate locations in space. To illustrate this, consider the situation depicted in Figure 1. If we shake a charge at r1 then a charge at location r2 will respond. But how did this action travel from r1 to r2? Various explanations were developed over the years, for example, by postulating an aether that fills all space and that acts as a transport medium, similar to water waves. The fields E and B are pure constructs to deal with the “action-at-distance’ problem. Thus, forces generated by ? q 1 q2 r 1 r2 Figure 1: Illustration of “action-at-distance”. Shaking a charge at r1 makes a sec- ond charge at r2 respond. 1 2 electrical charges and currents are explained in terms of E and B, quantities that we cannot measure directly. -

Lecture 4 Plane Waves 3D Differential Wave Equation Spherical Waves Cylindrical Waves
Lecture 4 Chapter 2 Wave Motion Plane waves 3D Differential wave equation Spherical waves Cylindrical waves 3-D waves: plane waves (simplest 3-D waves) All the surfaces of constant phase of disturbance form parallel planes that are perpendicular to the propagation direction 3-D waves: plane waves (simplest 3-D waves) All the surfaces of constant phase of disturbance form parallel planes that are perpendicular to the propagation direction An equation of plane that is ˆ ˆ ˆ perpendicular to k kx i k y j kzk Unit vectors k r const a All possible coordinates of vector r are on a plane k Can construct a set of planes over which varies in space harmonically: r Asink r or r Acosk r or r Aeikr Plane waves r sink r The spatially repetitive nature can be expressed as: k r r k In exponential form: r Aeikr Aeikr k / k Aeikr eik For that to be true: ei2 1 k 2 2 k Vector k is called propagation vector Plane waves: equation r Aeik r This is snap-shot in time, no time dependence To make it move need to add time dependence the same way as for one-dimensional wave: r,t Aeik r t Plane wave equation Plane wave: propagation velocity Can simplify to 1-D case assuming that wave propagates along x: ˆ r || i ikxt r,t Aeikr t r,t Ae We have shown that for 1-D wave phase velocity is: That is true for any direction of k v + propagate with k k - propagate opposite to k More general case: see page 26 Example: two plane waves Same wavelength: k1= k2=k=2/, Write equations for both waves. -

Maxwell's Equations, Electromagnetic Waves, and Stokes Parameters B
MAXWELL’S EQUATIONS, ELECTROMAGNETIC WAVES, AND STOKES PARAMETERS MICHAEL I. MISHCHENKO AND LARRY D. TRAVIS NASA Goddard Institute for Space Studies, 2880 Broadway, New York, NY 10025, USA 1. Introduction The theoretical basis for describing elastic scattering of light by particles and surfaces is formed by classical electromagnetics. In order to make this volume sufficiently self-contained, this introductory chapter provides a summary of those concepts and equations of electromagnetic theory that will be used extensively in later chapters and introduces the necessary notation. We start by formulating the macroscopic Maxwell equations and constitutive relations and discussing the fundamental time-harmonic plane- wave solution that underlies the basic optical idea of a monochromatic parallel beam of light. This is followed by the introduction of the Stokes parameters and a discussion of their ellipsometric content. Then we consider the concept of a quasi-monochromatic beam of light and its implications and briefly discuss how the Stokes parameters of monochromatic and quasi- monochromatic light can be measured in practice. In the final two sections, we discuss another fundamental solution of Maxwell’s equations in the form of a time-harmonic outgoing spherical wave and introduce the concept of the coherency dyad, which plays a vital role in the theory of multiple light scattering by random particle ensembles. 2. Maxwell’s equations and constitutive relations The theory of classical optics phenomena is based on the set of four Maxwell’s equations for the macroscopic electromagnetic field at interior points in matter, which in SI units read: ∇ ⋅D(r, t) = ρ(r, t), (2.1) ∂B(r, t) ∇ ×E(r, t) = − , (2.2) ∂t ∇ ⋅B(r, t) = 0, (2.3) 1 G. -
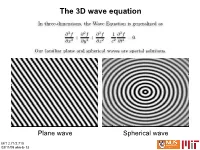
Introduction to Electromagnetics; Maxwell's Equations and Derivation of the Wave Equation for Light; Polarization Justificat
The 3D wave equation Plane wave Spherical wave MIT 2.71/2.710 03/11/09 wk6-b-13 Planar and Spherical Wavefronts Planar wavefront (plane wave): The wave phase is constant along a planar surface (the wavefront). As time evolves, the wavefronts propagate at the wave speed without changing; we say that the wavefronts are invariant to propagation in this case. Spherical wavefront (spherical wave): The wave phase is constant along a spherical surface (the wavefront). As time evolves, the wavefronts propagate at the wave speed and expand outwards while preserving the wave’s energy. MIT 2.71/2.710 03/11/09 wk6-b-14 Wavefronts, rays, and wave vectors Rays are: k 1) normals to the wavefront surfaces 2) trajectories of “particles of light” Wave vectors: At each point on the wavefront, we may assign a normal vector k This is known as the wave vector; k it magnitude k is the wave number and it is defined as k MIT 2.71/2.710 03/11/09 wk6-b-15 3D wave vector from the wave equation wavefront x kx k wave vector kz z ky y MIT 2.71/2.710 03/11/09 wk6 -b-16 3D wave vector and the Descartes sphere The wave vector represents the momentum of the wave. Consistent with Geometrical Optics, its magnitude is constrained to be proportional to the refractive index n (2π/λfree is a normalization factor) In wave optics, the Descartes sphere is also known as Ewald sphere or simply as the k-sphere. (Ewald sphere may be familiar to you from solid state physics) MIT 2.71/2.710 03/11/09 wk6-b-17 Spherical wave “point” source Outgoing rays Outgoing wavefronts (spherical) MIT 2.71/2.710 03/11/09 wk6-b-18 Dispersive waves Dispersion curves for glass Fig. -

Trapping of Resonant Metallic Nanoparticles with Engineered Vectorial Optical Field
Nanophotonics 2014; 3(6): 351–361 Research article Guanghao Rui and Qiwen Zhan* Trapping of resonant metallic nanoparticles with engineered vectorial optical field Abstract: Optical trapping and manipulation using optical trapping have evolved from simple manipulation focused laser beams has emerged as a powerful tool in the to the application of calibrated forces on, and the meas- biological and physical sciences. However, scaling this urement of nanometer-level displacement of optically technique to metallic nanoparticles remains challeng- trapped objects. Because of their unique features, optical ing due to the strong scattering force and optical heating tweezers have revolutionized the experimental study of effect. In this work, we propose a novel strategy to opti- small particles and become an important tool for research cally trap metallic nanoparticles even under the resonant in the fields of biology, physical chemistry and soft matter condition using engineered optical field. The distribution physics [2]. of the optical forces can be tailored through optimizing Nowadays, optical trapping has been successfully the spatial distribution of a vectorial optical illumina- implemented in two main size regimes: the sub-nanometer tion to favour the stable trapping of a variety of metallic (e.g., cooling of atoms, ions and molecules) and microm- nanoparticles under various conditions. It is shown that eter scale (such as cells). However, it has been difficult to this optical tweezers has the ability of generating negative apply these techniques to the nanoscale between ∼1 nm scattering force and supporting stable three-dimensional and 100 nm because of the challenges in scaling up the trapping for gold nanoparticles at resonance while avoid- techniques optimized for atom cooling, or scaling down ing trap destabilization due to optical overheating. -

Light Emission from Self‐Assembled and Laser‐Crystallized
Rapid #: -16480465 CROSS REF ID: 1104701 LENDER: ZCU :: Electronic BORROWER: PUL :: Interlibrary Services, Firestone TYPE: Article CC:CCG JOURNAL TITLE: Advanced optical materials USER JOURNAL TITLE: Advanced Optical Materials ARTICLE TITLE: Light Emission from Self-Assembled and Laser-Crystallized Chalcogenide Metasurface ARTICLE AUTHOR: Feifan Wang, Zi Wang, Dun Mao, Mingkun Chen, Qiu L VOLUME: 8 ISSUE: 8 MONTH: April YEAR: 2020 PAGES: NA ISSN: 2195-1071 OCLC #: Processed by RapidX: 8/17/2020 9:02:16 AM This material may be protected by copyright law (Title 17 U.S. Code) FULL PAPER www.advopticalmat.de Light Emission from Self-Assembled and Laser-Crystallized Chalcogenide Metasurface Feifan Wang, Zi Wang, Dun Mao, Mingkun Chen, Qiu Li, Thomas Kananen, Dustin Fang, Anishkumar Soman, Xiaoyong Hu, Craig B. Arnold,* and Tingyi Gu* demonstrated to improve the light emis- Subwavelength periodic confinement can collectively and selectively sion efficiency through enhancing light enhance local light intensity and enable control over the photoinduced phase outcoupling efficiency[1,2] and sponta- [3–5] transformations at the nanometer scale. Standard nanofabrication process neous emission rate. Nanophotonic engineering can lead to narrowband can result in geometrical and compositional inhomogeneities in optical phase and directive light emission.[6–8] Chal- change materials, especially chalcogenides, as those materials exhibit poor cogenide materials with unique phase chemical and thermal stability. Here the self-assembled planar chalcogenide change properties have been explored nanostructured array is demonstrated with resonance-enhanced light for tunable thermal emission[9] or reflec- [10] emission to create an all-dielectric optical metasurface, by taking advantage tion in infrared wavelength ranges.