Acrylonitrile Fact Sheet 051905 Final AQDB
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ECO-Ssls for Pahs
Ecological Soil Screening Levels for Polycyclic Aromatic Hydrocarbons (PAHs) Interim Final OSWER Directive 9285.7-78 U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 June 2007 This page intentionally left blank TABLE OF CONTENTS 1.0 INTRODUCTION .......................................................1 2.0 SUMMARY OF ECO-SSLs FOR PAHs......................................1 3.0 ECO-SSL FOR TERRESTRIAL PLANTS....................................4 5.0 ECO-SSL FOR AVIAN WILDLIFE.........................................8 6.0 ECO-SSL FOR MAMMALIAN WILDLIFE..................................8 6.1 Mammalian TRV ...................................................8 6.2 Estimation of Dose and Calculation of the Eco-SSL ........................9 7.0 REFERENCES .........................................................16 7.1 General PAH References ............................................16 7.2 References Used for Derivation of Plant and Soil Invertebrate Eco-SSLs ......17 7.3 References Rejected for Use in Derivation of Plant and Soil Invertebrate Eco-SSLs ...............................................................18 7.4 References Used in Derivation of Wildlife TRVs .........................25 7.5 References Rejected for Use in Derivation of Wildlife TRV ................28 i LIST OF TABLES Table 2.1 PAH Eco-SSLs (mg/kg dry weight in soil) ..............................4 Table 3.1 Plant Toxicity Data - PAHs ..........................................5 Table 4.1 -

Acrylonitrile
Common Name: ACRYLONITRILE CAS Number: 107-13-1 DOT Number: UN 1093 (Inhibited) RTK Substance number: 0024 DOT Hazard Class: 3 (Flammable Liquid) Date: May 1998 Revisions: December 2005 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY * Acrylonitrile can affect you when breathed in and by training concerning chemical hazards and controls. The federal passing through your skin. OSHA Hazard Communication Standard 29 CFR 1910.1200, * Acrylonitrile is a CARCINOGEN--HANDLE WITH requires private employers to provide similar training and EXTREME CAUTION. information to their employees. * Acrylonitrile should be handled as a TERATOGEN-- WITH EXTREME CAUTION. * Exposure to hazardous substances should be routinely * Skin contact can cause severe irritation and blistering. evaluated. This may include collecting personal and area air * Exposure to Acrylonitrile can irritate the eyes, nose, and samples. You can obtain copies of sampling results from throat. your employer. You have a legal right to this information * Breathing Acrylonitrile can irritate the lungs causing under the OSHA Standard 29 CFR 1910.1020. coughing and/or shortness of breath. Higher exposures can * If you think you are experiencing any work-related health cause a build-up of fluid in the lungs (pulmonary edema), a problems, see a doctor trained to recognize occupational medical emergency, with severe shortness of breath. diseases. Take this Fact Sheet with you. * Exposure to Acrylonitrile can cause weakness, headache, * ODOR THRESHOLD = 1.6 ppm. dizziness, confusion, nausea, vomiting, and can lead to * The range of accepted odor threshold values is quite broad. death. Caution should be used in relying on odor alone as a * Repeated exposure can irritate the nose causing discharge, warning of potentially hazardous exposures. -

Hydroxyacetonitrile (HOCH2CN) As a Precursor for Formylcyanide (CHOCN), Ketenimine (CH2CNH), and Cyanogen (NCCN) in Astrophysical Conditions
A&A 549, A93 (2013) Astronomy DOI: 10.1051/0004-6361/201219779 & c ESO 2013 Astrophysics Hydroxyacetonitrile (HOCH2CN) as a precursor for formylcyanide (CHOCN), ketenimine (CH2CNH), and cyanogen (NCCN) in astrophysical conditions G. Danger1, F. Duvernay1, P. Theulé1, F. Borget1, J.-C. Guillemin2, and T. Chiavassa1 1 Aix-Marseille Univ, CNRS, PIIM UMR 7345, 13397 Marseille, France e-mail: [email protected] 2 Institut des Sciences Chimiques de Rennes, École Nationale Supérieure de Chimie de Rennes, CNRS, UMR 6226, Avenue du Général Leclerc, CS 50837, 35708 Rennes Cedex 7, France Received 8 June 2012 / Accepted 19 November 2012 ABSTRACT Context. The reactivity in astrophysical environments can be investigated in the laboratory through experimental simulations, which provide understanding of the formation of specific molecules detected in the solid phase or in the gas phase of these environments. In this context, the most complex molecules are generally suggested to form at the surface of interstellar grains and to be released into the gas phase through thermal or non-thermal desorption, where they can be detected through rotational spectroscopy. Here, we focus our experiments on the photochemistry of hydroxyacetonitrile (HOCH2CN), whose formation has been shown to compete with aminomethanol (NH2CH2OH), a glycine precursor, through the Strecker synthesis. Aims. We present the first experimental investigation of the ultraviolet (UV) photochemistry of hydroxyacetonitrile (HOCH2CN) as a pure solid or diluted in water ice. Methods. We used Fourier transform infrared (FT-IR) spectroscopy to characterize photoproducts of hydroxyacetonitrile (HOCH2CN) and to determine the different photodegradation pathways of this compound. To improve the photoproduct identifications, irradiations of hydroxyacetonitrile 14N and 15N isotopologues were performed, coupled with theoretical calculations. -

Gouvernement Du Canada
50-1443-eng-cov.qxd 5/18/00 19:56 Page 1 Environment Environnement Canada Canada Health Santé Canada Canada Canadian Environmental Protection Act, 1999 PRIORITY SUBSTANCES LIST ASSESSMENT REPORT Acrylonitrile Canada 50-1443-eng-cov.qxd 5/18/00 19:56 Page 2 Canadian Cataloguing in Publication Data Priority substances list assessment report: acrylonitrile (Priority substances list assessment report) Issued also in French under title: Liste des substances d'intérêt prioritaire, rapport d'évalua- tion, acrylonitrile. Canadian Environmental Protection Act. Includes bibliographical references. Co-published by Health Canada. Issued also on the Internet. ISBN 0-662-28587-5 Cat. no. En40-215/49E 1. Acrylonitrile -- Toxicity testing -- Canada. 2. Acrylonitrile -- Environmental aspects -- Canada. 3. Environmental monitoring -- Canada. I. Canada. Environment Canada. II. Canada. Health Canada. III. Series. TD887.A37P74 2000 363.738'4 C00-980073-5 Additional information can be obtained at Environment Canadas Web site at www.ec.gc.ca or from the Inquiry Centre at 1-800-668-6767. © Minister of Public Works and Government Services 2000 50-1443-eng-cov.qxd 5/18/00 19:56 Page 3 Canadian Environmental Protection Act, 1999 PRIORITY SUBSTANCES LIST ASSESSMENT REPORT Acrylonitrile Environment Canada Health Canada May 2000 50-1443-eng.qxd 5/29/00 14:48 Page 1 TABLE OF CONTENTS SYNOPSIS .................................................................................................................... 1 1.0 INTRODUCTION ............................................................................................... -

39. Acrylonitrile アクリロニトリル
IPCS UNEP//ILO//WHO 国際化学物質簡潔評価文書 Concise International Chemical Assessment Document No.39 Acrylonitrile(2002) アクリロニトリル 世界保健機関 国際化学物質安全性計画 国立医薬品食品衛生研究所 安全情報部 2007 目 次 序 言 1. 要 約 ------------------------------------------------------------------------------------------- 5 2. 物質の特定および物理的・化学的性質 ----------------------------------------------- 7 3. 分析方法 -------------------------------------------------------------------------------------- 8 4. ヒトおよび環境の暴露源 ----------------------------------------------------------------- 10 4.1 自然界での発生源 ------------------------------------------------------------------------- 10 4.2 人為的発生源 ------------------------------------------------------------------------------- 10 4.3 生産と用途 ---------------------------------------------------------------------------------- 10 5. 環境中の移動・分布・変換 -------------------------------------------------------------- 11 5.1 大 気 ------------------------------------------------------------------------------------------ 11 5.2 水 ---------------------------------------------------------------------------------------------- 11 5.3 土壌と底質 ---------------------------------------------------------------------------------- 12 5.4 生物相 ---------------------------------------------------------------------------------------- 12 5.5 環境中分配 ---------------------------------------------------------------------------------- 13 6. 環境中の濃度とヒトの暴露量 ------------------------------------------------------------ 14 6.1 環境中の濃度 ------------------------------------------------------------------------------- 14 6.1.1 -

Polymer Effect on Molecular Recognition. Enhancement of Molecular-Shape Selectivity for Polycyclic Aromatic Hydrocarbons by Poly(Acrylonitrile)
Polymer Journal, Vol.34, No. 6, pp 437—442 (2002) Polymer Effect on Molecular Recognition. Enhancement of Molecular-Shape Selectivity for Polycyclic Aromatic Hydrocarbons by Poly(acrylonitrile) Makoto TAKAFUJI, Wei DONG, Yoshihiro GOTO, Toshihiko SAKURAI, ∗ † Shoji NAGAOKA, and Hirotaka IHARA Department of Applied Chemistry & Biochemistry, Faculty of Engineering, Kumamoto University, Kumamoto 860–8555, Japan ∗Kumamoto Industrial Research Institute, Kumamoto 862–0901, Japan (Received December 10, 2001; Accepted April 25, 2002) ABSTRACT: Poly(acrylonitrile) immobilized onto porous silica (Sil-ANn) was prepared to evaluate the effect of polymerization degree of poly(acrylonitrile) on selective interaction with polycyclic aromatic hydrocarbons. The HPLC using the packed column (Sil-ANn) and an aqueous solution as a mobile phase showed higher selectivity for structural isomers of polycyclic aromatic hydrocarbons compared with simply cyanopropylated silica (Sil-CN) and alkylated silica (Sil-C4). It was considered that silica-supported poly(acrylonitrile) recognized molecular aromaticity of π-electron con- taining compounds rather than molecular hydrophobicity. Furthermore, similar results were obtained in the selectivity towards geometrical isomers such as trans- and cis-stilbenes or triphenylene and o-terphenyl and structural isomers such as o-, m-, p-terphenyls. Also the separation factors increased with an increase in polymerization degree of ANn. This paper discusses that polymeric structures enhance the selectivity. KEY WORDS Polymer Grafting -

Acrylonitrile
Acrylonitrile 107-13-1 Hazard Summary Exposure to acrylonitrile is primarily occupational: it is used in the manufacture of acrylic acid and modacrylic fibers. Acute (short-term) exposure of workers to acrylonitrile has been observed to cause mucous membrane irritation, headaches, dizziness, and nausea. No information is available on the reproductive or developmental effects of acrylonitrile in humans. Based on limited evidence in humans and evidence in rats, EPA has classified acrylonitrile as a probable human carcinogen (Group B1). Please Note: The main sources of information for this fact sheet are EPA's Integrated Risk Information System (IRIS) (4), which contains information on inhalation chronic toxicity of acrylonitrile and the RfC and the carcinogenic effects of acrylonitrile including the unit cancer risk for inhalation exposure, EPA's Health Effects Assessment for Acrylonitrile (6), and the Agency for Toxic Substances and Disease Registry's (ATSDR's) Toxicological Profile for Acrylonitrile (1). Uses Acrylonitrile is primarily used in the manufacture of acrylic and modacrylic fibers. It is also used as a raw material in the manufacture of plastics (acrylonitrile-butadiene-styrene and styrene-acrylonitrile resins), adiponitrile, acrylamide, and nitrile rubbers and barrier resins. (1,6) Sources and Potential Exposure Human exposure to acrylonitrile appears to be primarily occupational, via inhalation. (1) Acrylonitrile may be released to the ambient air during its manufacture and use. (1) Assessing Personal Exposure Acrylonitrile -

Toxicological Profile for Acetone Draft for Public Comment
ACETONE 1 Toxicological Profile for Acetone Draft for Public Comment July 2021 ***DRAFT FOR PUBLIC COMMENT*** ACETONE ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. This information is distributed solely for the purpose of pre dissemination public comment under applicable information quality guidelines. It has not been formally disseminated by the Agency for Toxic Substances and Disease Registry. It does not represent and should not be construed to represent any agency determination or policy. ***DRAFT FOR PUBLIC COMMENT*** ACETONE iii FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. -

Ethylene Oxide
ETHYLENE OXIDE Ethylene oxide was considered by previous IARC Working Groups in 1976, 1984, 1987, 1994, and 2007 (IARC, 1976, 1985, 1987, 1994, 2008). Since that time new data have become avail- able, which have been incorporated in this Monograph, and taken into consideration in the present evaluation. 1. Exposure Data 1.2 Uses Ethylene oxide is an important raw material 1.1 Identification of the agent used in the manufacture of chemical derivatives From IARC (2008), unless indicated otherwise that are the basis for major consumer goods in Chem. Abstr. Serv. Reg. No.: 75-21-8 virtually all industrialized countries. More than Chem. Abstr. Serv. Name: Oxirane half of the ethylene oxide produced worldwide Synonyms: 1,2-Epoxyethane is used in the manufacture of mono-ethylene glycol. Conversion of ethylene oxide to ethylene O glycols represents a major use for ethylene oxide in most regions: North America (65%), western Europe (44%), Japan (63%), China (68%), Other Asia (94%), and the Middle East (99%). Important C2H4O Relative molecular mass: 44.06 derivatives of ethylene oxide include di-ethylene Description: Colourless, flammable gas glycol, tri-ethylene glycol, poly(ethylene) (O’Neill, 2006) glycols, ethylene glycol ethers, ethanol-amines, Boiling-point: 10.6 °C (Lide, 2008) and ethoxylation products of fatty alcohols, Solubility: Soluble in water, acetone, fatty amines, alkyl phenols, cellulose and benzene, diethyl ether, and ethanol (Lide, poly(propylene) glycol (Occupational Safety and 2008) Health Administration, 2005; Devanney, 2010). Conversion factor: mg/m3 = 1.80 × ppm; A very small proportion (0.05%) of the annual calculated from: mg/m3 = (relative production of ethylene oxide is used directly in molecular weight/24.45) × ppm, assuming the gaseous form as a sterilizing agent, fumigant standard temperature (25 °C) and pressure and insecticide, either alone or in non-explo- (101.3 kPa). -

Naphthalene Photoinitiated Copolymerizafion of Unsaturated Monomers
Naphthalene photoinitiated copolymerizafion of unsaturated monomers J. BARTOŇ, I. ČAPEK, D. LATH, and E. LATHOVÁ Polymer Institute, Slovak Academy of Sciences, 809 34 Bratislava Received 18 February 1976 For the naphthalene photoinitiated copolymerization of acrylonitrile with styrene, methyl methacrylate, vinyl chloride, vinyl acetate, and isoprene the copolymerization parameters, number average molecular weights, and limiting viscosity numbers of copolymers were determined. It was found that copolyme rization rates increased by increasing the acrylonitrile concentration in feed. The results obtained point at the radical nature of copolymerization. При нафталином фотоинициированной сополимеризации акрилонитри- ла со стиролом, метилметакрилатом, винилхлоридом, винилацетатом и изопреном были установлены параметры сополимеризации, средний молекулярный вес и предельная характеристика вязкости сополимеров. Было установлено, что скорость сополимеризации увеличивается с повы шением концентрации акрилонитрила в затравке. Полученные результа ты указывают на радикальную сущность сополимеризации. In our papers [1—3] we have shown that aromatic hydrocarbons photoinitiated the polymerization of acrylonitrile. It was concluded that polymerization proceeds by a free radical mechanism. As initiating particles the ion radicals were con sidered. These initiating particles were formed in the subsequent reactions of the reaction product arising in the interaction between aromatic hydrocarbon in the first excited singlet state and acrylonitrile in the ground state. In this communication we report the results of study of copolymerization of acrylonitrile (AN) with methyl methacrylate (MMA), styrene (S), vinyl chloride (VC1), vinyl acetate (VAc), and isoprene (I) in the presence of naphthalene (N) acting as photoinitiator. Experimental Monomers, all commercial products were carefully purified by three times repeated rectification under reduced pressure of argon. Vinyl chloride was dried by passing it through silica gel column. -

Acrylonitrile
ACRYLONITRILE This substance was considered by previous Working Groups, in February 1978 (IARC, 1979) and March 1987 (IARC, 1987a). Since that time, new data have become available, and these have been incorporated into the monograph and taken into consi- deration in the present evaluation. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Nomenclature Chem. Abstr. Serv. Reg. No.: 107-13-1 Chem. Abstr. Name: 2-Propenenitrile Synonyms: AN; cyanoethylene; propenenitrile; VCN; vinyl cyanide 1.1.2 Structural and molecular formulae and relative molecular mass H2 CCHCN C3H3N Relative molecular mass: 53.06 1.1.3 Chemical and physical properties of the pure substance (a) Description: Colourless liquid (Verschueren, 1996) (b) Boiling-point: 77.3°C (Lide, 1995) (c) Melting-point: –83.5°C (Lide, 1995) 20 (d) Density: d4 0.8060 (Lide, 1995) (e) Spectroscopy data: Infrared, nuclear magnetic resonance and mass spectral data have been reported (Sadtler Research Laboratories, 1980; Brazdil, 1991) (f) Solubility: Soluble in water (7.35 mL/100 mL at 20°C); very soluble in acetone, benzene, diethyl ether and ethanol (Lide, 1995; Budavari, 1996) (g) Volatility: Vapour pressure, 13.3 kPa at 23°C; relative vapour density (air = 1), 1.83 (Verschueren, 1996) (h) Stability: Flash-point (open cup), 0°C; flammable; polymerizes spontaneously, particularly in the absence of oxygen, on exposure to visible light and in contact with concentrated alkali (Budavari, 1996) (i) Explosive limits: Lower, 3.05%; upper, 17.0% (Budavari, 1996) (j) Octanol/water partition coefficient (P): log P, 0.25 (Hansch et al., 1995) –43– 44 IARC MONOGRAPHS VOLUME 71 (k) Conversion factor: mg/m3 = 2.17 × ppm1 1.1.4 Technical products and impurities Acrylonitrile of 99.5–99.7% purity is available commercially, with the following specifications (ppm by weight, maximum): acidity (as acetic acid), 10; acetone, 75; ace- tonitrile, 300; acrolein, 1; hydrogen cyanide, 5; total iron, 0.1; oxazole, 10; peroxides (as hydrogen peroxide), 0.2; water, 0.5%; and nonvolatile matter, 100. -
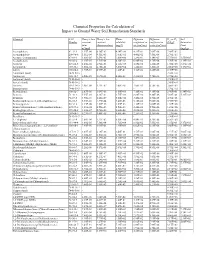
Chemical Properties Table
Chemical Properties for Calculation of Impact to Ground Water Soil Remediation Standards Chemical CAS Henry’s law Henry’s law Water Diffusion Diffusion Koc or Kd Soil Number constant constant solubility coefficient in coefficient in (L/kg)a Saturation (atm- (dimensionless) (mg/L) air (cm2/sec) water (cm2/sec) Limit m3/mol) (mg/kg) Acenaphthene 83-32-9 1.55E-04 6.36E-03 4.24E+00 4.21E-02 7.69E-06 7.08E+03 - Acenaphthylene 208-96-8 1.11E-04 4.51E-03 1.60E+01 4.40E-02 7.50E-06 2.76E+03 - Acetone (2-propanone) 67-64-1 3.88E-05 1.59E-03 1.00E+06 1.24E-01 1.14E-05 5.75E-01 1.55E+05 Acetophenone 98-86-2 1.10E-05 4.51E-04 6.10E+03 6.00E-02 8.70E-06 3.70E+01 1.39E+03 Acrolein 107-02-8 1.20E-04 4.92E-03 2.10E+05 1.05E-01 1.20E-05 1.00E+00 3.27E+04 Acrylonitrile 107-13-1 1.00E-04 4.10E-03 7.40E+04 1.22E-01 1.30E-05 2.00E+00 1.17E+04 Aldrin 309-00-2 1.70E-04 6.97E-03 1.80E-01 1.32E-02 4.86E-06 2.45E+06 - Aluminum (total) 7429-90-5 - - - - - 1.50E+03 - Anthracene 120-12-7 6.50E-05 2.67E-03 4.34E-02 3.24E-02 7.74E-06 2.95E+04 - Antimony (total) 7440-36-0 - - - - - 4.50E+01 - Arsenic (total) 7440-38-2 - - - - - 2.60E+01 - Atrazine 1912-24-9 2.96E-09 1.21E-07 7.00E+01 2.60E-02 6.70E-06 3.60E+02 - Barium (total) 7440-39-3 - - - - - 1.70E+01 - Benzaldehyde 100-52-7 2.67E-05 1.09E-03 3.00E+03 7.30E-02 9.10E-06 2.90E+01 6.34E+02 Benzene 71-43-2 5.55E-03 2.28E-01 1.75E+03 8.80E-02 9.80E-06 5.89E+01 5.22E+02 Benzidine 92-87-5 3.90E-11 1.60E-09 5.00E+02 3.40E-02 1.50E-05 4.70E+01 - Benzo(a)anthracene (1,2-Benzanthracene) 56-55-3 3.35E-06 1.37E-04 9.40E-03 5.10E-02