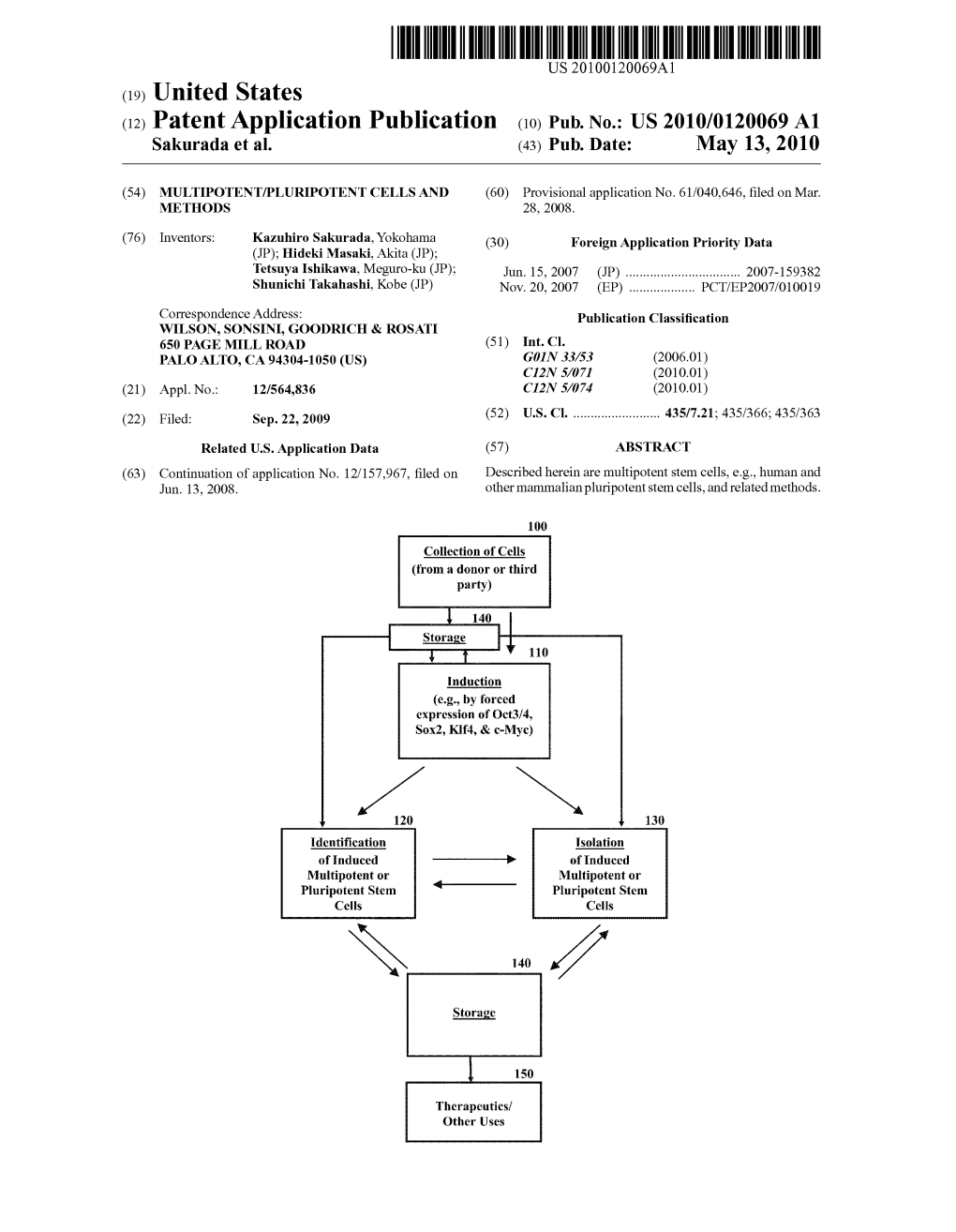
(12) Patent Application Publication (10) Pub. No.: US 2010/0120069 A1 Sakurada Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Involvement of DPP9 in Gene Fusions in Serous Ovarian Carcinoma
Smebye et al. BMC Cancer (2017) 17:642 DOI 10.1186/s12885-017-3625-6 RESEARCH ARTICLE Open Access Involvement of DPP9 in gene fusions in serous ovarian carcinoma Marianne Lislerud Smebye1,2, Antonio Agostini1,2, Bjarne Johannessen2,3, Jim Thorsen1,2, Ben Davidson4,5, Claes Göran Tropé6, Sverre Heim1,2,5, Rolf Inge Skotheim2,3 and Francesca Micci1,2* Abstract Background: A fusion gene is a hybrid gene consisting of parts from two previously independent genes. Chromosomal rearrangements leading to gene breakage are frequent in high-grade serous ovarian carcinomas and have been reported as a common mechanism for inactivating tumor suppressor genes. However, no fusion genes have been repeatedly reported to be recurrent driver events in ovarian carcinogenesis. We combined genomic and transcriptomic information to identify novel fusion gene candidates and aberrantly expressed genes in ovarian carcinomas. Methods: Examined were 19 previously karyotyped ovarian carcinomas (18 of the serous histotype and one undifferentiated). First, karyotypic aberrations were compared to fusion gene candidates identified by RNA sequencing (RNA-seq). In addition, we used exon-level gene expression microarrays as a screening tool to identify aberrantly expressed genes possibly involved in gene fusion events, and compared the findings to the RNA-seq data. Results: We found a DPP9-PPP6R3 fusion transcript in one tumor showing a matching genomic 11;19-translocation. Another tumor had a rearrangement of DPP9 with PLIN3. Both rearrangements were associated with diminished expression of the 3′ end of DPP9 corresponding to the breakpoints identified by RNA-seq. For the exon-level expression analysis, candidate fusion partner genes were ranked according to deviating expression compared to the median of the sample set. -

Role and Regulation of the P53-Homolog P73 in the Transformation of Normal Human Fibroblasts
Role and regulation of the p53-homolog p73 in the transformation of normal human fibroblasts Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Bayerischen Julius-Maximilians-Universität Würzburg vorgelegt von Lars Hofmann aus Aschaffenburg Würzburg 2007 Eingereicht am Mitglieder der Promotionskommission: Vorsitzender: Prof. Dr. Dr. Martin J. Müller Gutachter: Prof. Dr. Michael P. Schön Gutachter : Prof. Dr. Georg Krohne Tag des Promotionskolloquiums: Doktorurkunde ausgehändigt am Erklärung Hiermit erkläre ich, dass ich die vorliegende Arbeit selbständig angefertigt und keine anderen als die angegebenen Hilfsmittel und Quellen verwendet habe. Diese Arbeit wurde weder in gleicher noch in ähnlicher Form in einem anderen Prüfungsverfahren vorgelegt. Ich habe früher, außer den mit dem Zulassungsgesuch urkundlichen Graden, keine weiteren akademischen Grade erworben und zu erwerben gesucht. Würzburg, Lars Hofmann Content SUMMARY ................................................................................................................ IV ZUSAMMENFASSUNG ............................................................................................. V 1. INTRODUCTION ................................................................................................. 1 1.1. Molecular basics of cancer .......................................................................................... 1 1.2. Early research on tumorigenesis ................................................................................. 3 1.3. Developing -

Stem Cells® Original Article
® Stem Cells Original Article Properties of Pluripotent Human Embryonic Stem Cells BG01 and BG02 XIANMIN ZENG,a TAKUMI MIURA,b YONGQUAN LUO,b BHASKAR BHATTACHARYA,c BRIAN CONDIE,d JIA CHEN,a IRENE GINIS,b IAN LYONS,d JOSEF MEJIDO,c RAJ K. PURI,c MAHENDRA S. RAO,b WILLIAM J. FREEDa aCellular Neurobiology Research Branch, National Institute on Drug Abuse, Department of Health and Human Services (DHHS), Baltimore, Maryland, USA; bLaboratory of Neuroscience, National Institute of Aging, DHHS, Baltimore, Maryland, USA; cLaboratory of Molecular Tumor Biology, Division of Cellular and Gene Therapies, Center for Biologics Evaluation and Research, Food and Drug Administration, Bethesda, Maryland, USA; dBresaGen Inc., Athens, Georgia, USA Key Words. Embryonic stem cells · Differentiation · Microarray ABSTRACT Human ES (hES) cell lines have only recently been compared with pooled human RNA. Ninety-two of these generated, and differences between human and mouse genes were also highly expressed in four other hES lines ES cells have been identified. In this manuscript we (TE05, GE01, GE09, and pooled samples derived from describe the properties of two human ES cell lines, GE01, GE09, and GE07). Included in the list are genes BG01 and BG02. By immunocytochemistry and reverse involved in cell signaling and development, metabolism, transcription polymerase chain reaction, undifferenti- transcription regulation, and many hypothetical pro- ated cells expressed markers that are characteristic of teins. Two focused arrays designed to examine tran- ES cells, including SSEA-3, SSEA-4, TRA-1-60, TRA-1- scripts associated with stem cells and with the 81, and OCT-3/4. Both cell lines were readily main- transforming growth factor-β superfamily were tained in an undifferentiated state and could employed to examine differentially expressed genes. -

Márcio Lorencini Avaliação Global De Transcritos Associados Ao Envelhecimento Da Epiderme Humana Utilizando Microarranjos De
MÁRCIO LORENCINI AVALIAÇÃO GLOBAL DE TRANSCRITOS ASSOCIADOS AO ENVELHECIMENTO DA EPIDERME HUMANA UTILIZANDO MICROARRANJOS DE DNA GLOBAL EVALUATION OF TRANSCRIPTS ASSOCIATED TO HUMAN EPIDERMAL AGING WITH DNA MICROARRAYS CAMPINAS 2014 i ii UNIVERSIDADE ESTADUAL DE CAMPINAS Instituto de Biologia MÁRCIO LORENCINI AVALIAÇÃO GLOBAL DE TRANSCRITOS ASSOCIADOS AO ENVELHECIMENTO DA EPIDERME HUMANA UTILIZANDO MICROARRANJOS DE DNA GLOBAL EVALUATION OF TRANSCRIPTS ASSOCIATED TO HUMAN EPIDERMAL AGING WITH DNA MICROARRAYS Tese apresentada ao Instituto de Biologia da Universidade Estadual de Campinas como parte dos requisitos exigidos para a obtenção do título de Doutor em Genética e Biologia Molecular, na área de Genética Animal e Evolução. Thesis presented to the Institute of Biology of the University of Campinas in partial fulfillment of the requirements for the degree of Doctor in Genetics and Molecular Biology, in the area of Animal Genetics and Evolution. Orientador/Supervisor: PROF. DR. NILSON IVO TONIN ZANCHIN ESTE EXEMPLAR CORRESPONDE À VERSÃO FINAL DA TESE DEFENDIDA PELO ALUNO MÁRCIO LORENCINI, E ORIENTADA PELO PROF. DR. NILSON IVO TONIN ZANCHIN. ________________________________________ Prof. Dr. Nilson Ivo Tonin Zanchin CAMPINAS 2014 iii iv COMISSÃO JULGADORA 31 de janeiro de 2014 Membros titulares: Prof. Dr. Nilson Ivo Tonin Zanchin (Orientador) __________________________ Assinatura Prof. Dr. José Andrés Yunes __________________________ Assinatura Profa. Dra. Maricilda Palandi de Mello __________________________ Assinatura Profa. Dra. Bettina -

Prediction of the Coding Sequences of Unidentified Human Genes. XXII
DNA Research 8, 319–327 (2001) Short Communication Prediction of the Coding Sequences of Unidentified Human Genes. XXII. The Complete Sequences of 50 New cDNA Clones Which Code for Large Proteins Takahiro Nagase,∗ Reiko Kikuno, and Osamu Ohara Kazusa DNA Research Institute, 1532-3 Yana, Kisarazu, Chiba 292-0812, Japan (Received 3 December 2001) Abstract As an extension of human cDNA projects for accumulating sequence information on the coding se- Downloaded from quences ofunidentified genes, we herein present the entire sequences of50 cDNA clones, named KIAA1939– KIAA1988. cDNA clones to be entirely sequenced were selected by two approaches based on their protein-coding potentialities prior to sequencing: 10 cDNA clones were chosen because their encoding proteins had a molecular mass larger than 50 kDa in an in vitro transcription/translation system; the re- maining 40 cDNA clones were selected because their putative proteins—as determined by analysis ofthe http://dnaresearch.oxfordjournals.org/ genomic sequences flanked by both the terminal sequences ofcDNAs using the GENSCAN gene prediction program—were larger than 400 amino acid residues. According to the sequence data, the average sizes of the inserts and corresponding open reading frames of cDNA clones analyzed here were 4.6 kb and 1.9 kb (643 amino acid residues), respectively. From the results ofhomology and motifsearches against the public databases, the functionalcategories ofthe 31 predicted gene products could be assigned; 25 ofthese pre- dicted gene products (81%) were classified into proteins relating to cell signaling/communication, nucleic acid management, and cell structure/motility. The expression profiles ofthe genes were also studied in 10 human tissues, 8 brain regions, spinal cord, fetal brain and fetal liver by reverse transcription-coupled polymerase chain reaction, the products ofwhich were quantified by enzyme-linked immunosorbent assay. -

Next-Generation Sequencing Approach For
UNIVERSITÀ DEGLI STUDI DI MILANO Scuola di Dottorato in Medicina Molecolare ! Ciclo XXVII Anno Accademico 2013/2014 NEXT-GENERATION SEQUENCING APPROACH FOR TRANSCRIPTOME AND EPIGENOME DEFINITION OF HUMAN HEMATOPOIETIC STEM/PROGENITOR CELLS AND THEIR EARLY ERYTHROID AND MYELOID COMMITTED PROGENY Dottorando: Luca PETITI Direttore della Scuola di Dottorato: Prof. Mario CLERICI! Tutore: Prof. Cristina BATTAGLIA Co-tutore: Dr. Clelia PEANO UNIVERSITÀ DEGLI STUDI DI MILANO Scuola di Dottorato in Medicina Molecolare ! Ciclo XXVII Anno Accademico 2013/2014 Settore BIO/10 Next-Generation Sequencing Approach for Transcriptome and Epigenome Definition of Human Hematopoietic Stem/Progenitor Cells and their Early Erythroid and Myeloid Committed Progeny Dottorando: Luca PETITI Matricola N° R09618 TUTORE: Prof.ssa Cristina BATTAGLIA CO-TUTORE: Dott.ssa Clelia PEANO DIRETTORE DEL DOTTORATO: Prof. Mario CLERICI! ABSTRACT Somatic stem cells are the basic tools of regenerative medicine and gene therapy, providing unique opportunities for the therapy of genetic and acquired disorders. The molecular mechanisms underlying fundamental characteristics of human somatic stem cells, such as self-renewal, commitment and differentiation, are still poorly understood. A better knowledge of these mechanisms is crucial to the understanding of stem cell biology and to the development of stem cell-based therapies. High-throughput approaches, such as next-generation sequencing technologies (NGS) became fundamental to study the transcriptome, the epigenome and the usage -

Molecular Genetic Characterization of Ovarian Tumors
Molecular genetic characterization of ovarian tumors Thesis for the degree of Philosophiae Doctor (PhD) University of Oslo, 2018 Antonio Agostini Section for Cancer Cytogenetics Institute for Cancer Genetics and Informatics The Norwegian Radium Hospital Oslo University Hospital Department of Biosciences Faculty of Mathematics and Natural Sciences University of Oslo The Norwegian Cancer Society Radium Hospital Foundation © Antonio Agostini, 2018 Series of dissertations submitted to the Faculty of Mathematics and Natural Sciences, University of Oslo No. 1992 ISSN 1501-7710 All rights reserved. No part of this publication may be reproduced or transmitted, in any form or by any means, without permission. Cover: Hanne Baadsgaard Utigard. Print production: Reprosentralen, University of Oslo. Molecular genetic characterization of ovarian tumors Antonio Agostini University of Oslo 2018 1 Cover Illustration Bellerophon fighting the Chimera, Roman mosaic, 1st century A.D. (Image from Wikimedia commons) Bellerophon was one of the most ancient heroes of Greek mythology and a monster hunter. One day King Iobates gave Bellerophon an impossible quest: to hunt the Chimera, a fire- breathing monster. The Chimera was truly ferocious, and Bellerophon could not harm the monster even if he was riding Pegasus, the winged horse. Feeling the heat of the breath of the Chimera, an idea struck him: He got a large block of lead that he mounted on his spear. Then he flew head-on towards the Chimera and managed to lodge the block of lead inside the monster's jaws. The lead melted in the monster’s throat and killed the Chimera. Finally Bellerophon returned victorious to King Iobates. Nora Heisterkamp and colleagues in 1983 described the first chimeric, cancer-specific gene, BCR-ABL1, originating from the translocation t(9;22)(q34;11) in chronic myeloid leukemia. -

A Network Analysis Based Proteomic and Transcriptomic Investigation Into HIV-Tat Induced Neuronal Dysfunction and the Neuroprotective Effect of Lithium
A network analysis based proteomic and transcriptomic investigation into HIV-Tat induced neuronal dysfunction and the neuroprotective effect of lithium By Tariq Ahmad Ganief University of Cape Town GNFTAR001 Dissertation submitted for the degree of Doctor of Philosophy In the Department of Integrative Biomedical Sciences University of Cape Town October 2015 Supervisor: Professor Jonathan M. Blackburn 1 The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town Declaration I, Tariq Ahmad Ganief, declare that this thesis is my own work (except where acknowledgements indicate otherwise). Neither the whole work nor part thereof has been, is being, or is to be submitted for any degree or examination at any other university. I empower the University of Cape Town to reproduce for the purposes of research either the whole or any part of the contents of this thesis, in any manner whatsoever. Signature of candidate: Tariq Ganief Signed on the 28 day of October, 2015 2 | P a g e Acknowledgements During the years of my PhD, I have received a tremendous amount of support both personally and professionally; without which, this road would have proved significantly more arduous. While words are an abysmal return for all your friendship and counsel, please do accept them, even if only as an acknowledgement in gratitude. -

Universidade De Lisboa Faculdade De Ciências Departamento De Química E Bioquímica
Universidade de Lisboa Faculdade de Ciências Departamento de Química e Bioquímica Molecular characterisation of the poorly differentiated and undifferentiated thyroid carcinomas using genome-wide approaches Jaime Miguel Gomes Pita Doutoramento em Bioquímica Especialidade em Genética Molecular 2013 Universidade de Lisboa Faculdade de Ciências Departamento de Química e Bioquímica Molecular characterisation of the poorly differentiated and undifferentiated thyroid carcinomas using genome-wide approaches Jaime Miguel Gomes Pita Tese orientada pelo Professor Doutor Valeriano Alberto Leite (Instituto Português de Oncologia de Lisboa, Francisco Gentil) e Professora Doutora Maria Luísa Cyrne (Faculdade de Ciências da Universidade de Lisboa) especialmente elaborada para a obtenção do grau de doutor em Bioquímica, especialidade em Genética Molecular 2013 A presente dissertação foi redigida de acordo com o disposto no artigo 45.º do Despacho n.º 4624/2012 do Diário da República 2.ª série - N.º 65 - 30 de Março de 2012 * * * As opiniões expressas nesta publicação são da exclusiva responsabilidade do seu autor. (...) (...) Eles não sabem que o sonho Eles não sabem, nem sonham, é vinho, é espuma, é fermento, que o sonho comanda a vida. bichinho álacre e sedento, Que sempre que um homem sonha de focinho pontiagudo, o mundo pula e avança que fossa através de tudo como bola colorida num perpétuo movimento. entre as mãos de uma criança. António Gedeão, Pedra Filosofal in ‘Movimento Perpétuo’ (1956) ‘The deeper we search the more we find there is to know, and as long as human life exists, I believe it will always be so.’ Albert Einstein Agradecimentos Agradecimentos Na presente tese de doutoramento são apresentados os resultados do trabalho de investigação realizado no Grupo de Endocrinologia Molecular da Unidade de Investigação de Patobiologia Molecular (UIPM) no Instituto Português de Oncologia de Lisboa, Francisco Gentil (IPOLFG) entre Janeiro de 2009 e Dezembro de 2012, sob a orientação do Professor Doutor Valeriano Alberto Leite e co-orientação da Doutora Branca Maria Cavaco. -

System Biology of Alcoholism: Understanding of the Consequences of the Metabolism in Brain Cells
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Sydney eScholarship SYSTEM BIOLOGY OF ALCOHOLISM: UNDERSTANDING OF THE CONSEQUENCES OF THE METABOLISM IN BRAIN CELLS 1 SYSTEM BIOLOGY OF ALCOHOLISM: UNDERSTANDING OF THE CONSEQUENCES OF THE METABOLISM IN BRAIN CELLS Mohammed Abul KASHEM A thesis submitted in fulfillment of the requirements for the degree of Doctor of Philosophy Discipline of Anatomy and Histology, University of Sydney November 2017 2 Contents Summary --------------------------------------------------------------------------------------------------- 8 Chapter 1 Introduction and aims ----------------------------------------------------------------------------------- 12 Chapter 2 Metabolomics of Neurotransmitters and Related Metabolites in Most-mortem Tissue from the Dorsal and Ventral Striatum of Alcoholic Human Brain Abstract ------------------------------------------------------------------------------------------------- 20 Introduction ----------------------------------------------------------------------------------------------- 21 Materials and Methods ------------------------------------------------------------------------------------ 24 Results ----------------------------------------------------------------------------------------------------- 29 Discussion ------------------------------------------------------------------------------------------------- 37 References ------------------------------------------------------------------------------------------------ 42 Chapter -

Blood Transcriptome Profiling in Myasthenia Gravis Patients to Assess Disease Activity: a Pilot RNA-Seq Study
http://dx.doi.org/10.5607/en.2016.25.1.40 Exp Neurobiol. 2016 Feb;25(1):40-47. pISSN 1226-2560 • eISSN 2093-8144 Original Article Blood Transcriptome Profiling in Myasthenia Gravis Patients to Assess Disease Activity: A Pilot RNA-seq Study Kee Hong Park1, Junghee Jung2, Jung-Hee Lee3 and Yoon-Ho Hong4* 1Department of Neurology, Gyeongsang National University Hospital, Jinju 52727, 2Department of Bioinformatics, Macrogen Inc., Seoul 08511, 3Department of Biomedical Science, Hallym University, Chuncheon 24252, 4Department of Neurology, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul 07061, Korea Myasthenia gravis (MG) is an antibody-mediated autoimmune disease characterized by exertional weakness. There is no biomarker to reflect disease activity and guide treatment decision. Here, we reported a pilot blood transcriptome study using RNA sequencing (RNA-seq) that identified differences of 5 samples in active status and 5 in remission from 8 different patients and 2 patients provided samples for both active and remission phase. We found a total of 28 differentially expressed genes (DEGs) possibly related to disease activity (23 up-regulated and 5 down-regulated). The DEGs were enriched for the cell motion and cell migration processes in which included were ICAM1, CCL3, S100P and GAB2. The apoptosis and cell death pathway was also significantly enriched, which includes NFKBIA, ZC3H12A, TNFAIP3, and PPP1R15A. Our result suggests that transcript abundance profiles of the genes involved in cell trafficking and apoptosis may be a molecular signature of the disease activity in MG patients. Key words: Transcriptome, RNA sequencing, myasthenia gravis, cell migration, apoptosis INTRODUCTION in approximately half of the generalized seronegative MG patients [2]. -

Characterization of the MIR23A Cluster in Diffuse Large B Cell Lymphoma
Characterization of the MIR23A Cluster in Diffuse Large B Cell Lymphoma Regulation and Targetome Identification Doctoral Thesis In partial fulfillment of the requirements for the degree “Doctor rerum naturalium (Dr. rer. nat.)“ in the Molecular Medicine Study Program at the Georg-August University Göttingen submitted by Natalie Veronika Freytag born in Zabrze, Poland Göttingen 2017 Members of the Thesis Committee: Prof. Dr. Dieter Kube, Supervisor Department of Haematology and Oncology University Medical Center Göttingen Prof. Dr. Peter Burfeind, Reviewer Department of Human Genetics University Medical Center Göttingen Halyna Shcherbata, PhD, Reviewer Max Planck Research Group for Gene Expression and Signaling Max Planck Institute for Biophysical Chemistry Göttingen Date of oral examination: 3.02.2017 Affidavit Here I declare that my doctoral thesis entitled “Characterization of the MIR23A Cluster in Diffuse Large B Cell Lymphoma - Regulation and Targetome Identification” has been writ- ten independently with no other sources and aids than quoted. Göttingen, October 2016 iii List of publications Annica Vlad-Fiegen, Natalie Veronika Freytag, Susanne Dorn, Oliver Müller, Sonja Eberth “The Wnt Pathway Target Gene CCND1 Changes Mitochondrial Localization and Decreases Mitochondrial Activity in Colorectal Cancer Cell Line SW480” Journal of Biosciences and Medicines, 2016, 4, 132-143 DOI: 10.4236/jbm.2016.412017 Natalie V. Freytag, Claudia Pommerenke, Yvonne Merkhoffer, Hilmar Quentmeier, Hans G. Drexler, Sonja Eberth “microRNAs encoded by the