Imaging of the Female Urethra
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Managing Female Urethral Diverticulum with a Standardized Technique Using a Pacifier-Trick Artifice to Facilitate Dissection
International Urogynecology Journal (2019) 30:789–794 https://doi.org/10.1007/s00192-018-3754-8 ORIGINAL ARTICLE Managing female urethral diverticulum with a standardized technique using a pacifier-trick artifice to facilitate dissection Philippe Neveü1 & Idir Ouzaid1 & Evanguelos Xylinas1 & Christophe Egrot1 & Vincent Ravery1 & Jean-François Hermieu1 Received: 21 February 2018 /Accepted: 12 August 2018 /Published online: 3 September 2018 # The International Urogynecological Association 2018 Abstract Introduction and hypothesis Managing urethral diverticula is challenging because of recurrence rate and postoperative compli- cations. Herein, we report a standardized, single-institution experience of surgical treatment of urethral diverticula in women. Methods The medical record of 37 female patients treated for urethral diverticula between 2005 and 2017 in a single institution were reviewed. All patients were operated in a standardized genupectoral position using a technical artifice called the pacifier trick to inflate diverticula throughout the procedure and facilitate its dissection. Symptoms at diagnosis, imaging findings, surgical parameters, postoperative complications, and recurrence rates were collected and are presented. Results Median age was 39 ± 11 (range 21–67) years. At diagnosis, recurrent urinary tract infections (UTI) (67%), vaginal mass (46%), pelvic pain (43%), dyspareunia (27%), and urinary incontinence (UI) (24%) were the most commonly reported symp- toms. Median operative time was 98 ± 31 (range 40–150) min. After a mean follow-up of 1 year, recurrence occurred in one (3%) patient. Immediate de novo postoperative UI decreased from 27% immediately after surgery to 3% after pelvic physical therapy. Pathological analyses found no malignant histology. Conclusions Surgical management of urethral diverticula in women is technically demanding. -

Urology & Incontinence
Urology & Incontinence - Glossary of Terms Anti-reflux Refers to a tube or collapsible material within a urine collection device to help prevent urine from reentering the tubing. Applicator collar Found on some Hollister male external catheters, a plastic guide with notches for the thumb and forefinger to assist proper placement against the tip of the penis. Aseptic intermittent catheterization The process of performing intermittent catheterization using sterile equipment and inserting the catheter in a sterile way. This would include a sterile ready-to-use product that can be inserted with gloves using a no-touch technique (e.g., the Advance Plus intermittent catheter or a VaPro hydrophilic catheter). Benzalkonium chloride (BZK) An antimicrobial solution used for cleansing the urethral opening prior to inserting an intermittent catheter. Does not stain skin or clothing. Bladder A collapsible balloon-like muscular organ that lies in the pelvis and functions to store and expel urine. Bladder catheterization A procedure in which a catheter is passed through the urethra or stoma into the bladder, usually for the purpose of draining urine. Bladder control The ability to control urination. Bladder diary A printed or electronic form to keep track of when one urinates or leaks urine. Catheter (urinary) A special type of hollow tube inserted through the urethra or a stoma to the bladder to withdraw urine or instill medication. Catheterization The process of inserting a tube into the bladder to drain urine. Clean intermittent catheterization The process of emptying the bladder using a clean intermittent catheter. It involves inserting and removing a catheter, typically several times a day. -
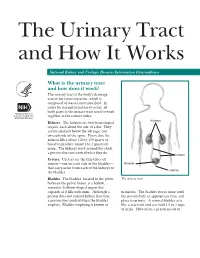
The Urinary Tract and How It Works
The Urinary Tract and How It Works National Kidney and Urologic Diseases Information Clearinghouse What is the urinary tract and how does it work? The urinary tract is the body’s drainage system for removing urine, which is composed of wastes and extra fluid. In order for normal urination to occur, all body parts in the urinary tract need to work together in the correct order. Kidneys Kidneys. The kidneys are two bean-shaped organs, each about the size of a fist. They are located just below the rib cage, one on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of blood to produce about 1 to 2 quarts of urine. The kidneys work around the clock; a person does not control what they do. Ureters Ureters. Ureters are the thin tubes of muscle—one on each side of the bladder— Bladder that carry urine from each of the kidneys to Urethra the bladder. Bladder. The bladder, located in the pelvis The urinary tract between the pelvic bones, is a hollow, muscular, balloon-shaped organ that expands as it fills with urine. Although a urination. The bladder stores urine until person does not control kidney function, the person finds an appropriate time and a person does control when the bladder place to urinate. A normal bladder acts empties. Bladder emptying is known as like a reservoir and can hold 1.5 to 2 cups of urine. How often a person needs to urinate depends on how quickly the kidneys Why is the urinary tract produce the urine that fills the bladder. -

15-1040-Junu Oh-Neuronal.Key
Neuronal Control of the Bladder Seung-June Oh, MD Department of urology, Seoul National University Hospital Seoul National University College of Medicine Contents Relevant end organs and nervous system Reflex pathways Implication in the sacral neuromodulation Urinary bladder ! body: detrusor ! trigone and bladder neck Urethral sphincters B Preprostatic S Smooth M. Sphincter Passive Prostatic S Skeletal M. Sphincter P Prostatic SS P-M Striated Sphincter Membraneous SS Periurethral Striated M. Pubococcygeous Spinal cord ! S2–S4 spinal cord ! primary parasympathetic micturition center ! bladder and distal urethral sphincter ! T11-L2 spinal cord ! sympathetic outflow ! bladder and proximal urethral sphincter Peripheral innervation ! The lower urinary tract is innervated by 3 principal sets of peripheral nerves: ! parasympathetic -pelvic n. ! sympathetic-hypogastric n. ! somatic nervous systems –pudendal n. ! Parasympathetic and sympathetic nervous systems form pelvic plexus at the lateral side of the rectum before reaching bladder and sphincter Sympathetic & parasympathetic systems ! Sympathetic pathways ! originate from the T11-L2 (sympathetic nucleus; intermediolateral column of gray matter) ! inhibiting the bladder body and excite the bladder base and proximal urethral sphincter ! Parasympathetic nerves ! emerge from the S2-4 (parasympathetic nucleus; intermediolateral column of gray matter) ! exciting the bladder and relax the urethra Sacral somatic system !emerge from the S2-4 (Onuf’s nucleus; ventral horn) !form pudendal nerve, providing -

Nerve Disease and Bladder Control
Nerve Disease and Bladder Control National Kidney and Urologic Diseases Information Clearinghouse For the urinary system to do its job, muscles and nerves must work together to hold Brain urine in the bladder and then release it at the right time. Nerves carry messages from NATIONAL the bladder to the brain to let it know when INSTITUTES the bladder is full. They also carry messages OF HEALTH Central nervous from the brain to the bladder, telling muscles system (brain either to tighten or release. A nerve prob and spinal cord) lem might affect your bladder control if the nerves that are supposed to carry messages Spinal cord between the brain and the bladder do not work properly. Nerve signals U.S. Department to bladder of Health and Bladder and sphincter Human Services What bladder control muscles problems does nerve damage cause? Nerves that work poorly can lead to three Urethra different kinds of bladder control problems. Overactive bladder. Damaged nerves may Sphincter muscles send signals to the bladder at the wrong time, causing its muscles to squeeze with Nerves carry signals from the brain to the bladder out warning. The symptoms of overactive and sphincter. bladder include • urinary frequency—defined as urination eight or more times a day or two or nerves to the sphincter muscles are dam more times at night aged, the muscles may become loose and allow leakage or stay tight when you are • urinary urgency—the sudden, strong trying to release urine. need to urinate immediately Urine retention. For some people, nerve • urge incontinence—leakage of urine damage means their bladder muscles do that follows a sudden, strong urge to not get the message that it is time to release urinate urine or are too weak to completely empty Poor control of sphincter muscles. -
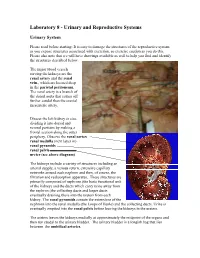
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder. -

Incomplete Bladder Emptying and Tips to Help
A guide for patients about incomplete bladder emptying and tips to help What is incomplete bladder emptying? When the bladder doesn't empty properly this may cause numerous problems. One of the problems is known as overflow incontinence. This is when the bladder doesn’t empty and urine leaks out. You may not get the message to go to the toilet either. The bladder never empties completely so some residue is normal. You may find it difficult to start to pass water and that even when you have started; the flow is weak and slow. You might find that you dribble after you have finished passing water. Perhaps you dribble urine all the time, even without noticing. What causes incomplete bladder emptying? Incomplete bladder emptying occurs when the muscles of the bladder are not able to squeeze properly to empty the bladder. This can happen in cases where there may have been nerve or muscle damage, perhaps caused by injury, surgery, or disease such as Parkinson's disease, Multiple Sclerosis and Spina Bifida. Medication can also cause problems with bladder emptying. In some cases, it can be due to an obstruction which is making it more difficult for you to empty your bladder. This obstruction can be caused by an enlarged prostate in men, a kidney stone blocking the urethra, constipation, or stricture of the urethra in either men or women, which makes it difficult for urine to flow out of the bladder outlet. If you are not able to empty completely, your bladder and its muscles may become floppy over time. -

Obstruction of the Urinary Tract 2567
Chapter 540 ◆ Obstruction of the Urinary Tract 2567 Table 540-1 Types and Causes of Urinary Tract Obstruction LOCATION CAUSE Infundibula Congenital Calculi Inflammatory (tuberculosis) Traumatic Postsurgical Neoplastic Renal pelvis Congenital (infundibulopelvic stenosis) Inflammatory (tuberculosis) Calculi Neoplasia (Wilms tumor, neuroblastoma) Ureteropelvic junction Congenital stenosis Chapter 540 Calculi Neoplasia Inflammatory Obstruction of the Postsurgical Traumatic Ureter Congenital obstructive megaureter Urinary Tract Midureteral structure Jack S. Elder Ureteral ectopia Ureterocele Retrocaval ureter Ureteral fibroepithelial polyps Most childhood obstructive lesions are congenital, although urinary Ureteral valves tract obstruction can be caused by trauma, neoplasia, calculi, inflam- Calculi matory processes, or surgical procedures. Obstructive lesions occur at Postsurgical any level from the urethral meatus to the calyceal infundibula (Table Extrinsic compression 540-1). The pathophysiologic effects of obstruction depend on its level, Neoplasia (neuroblastoma, lymphoma, and other retroperitoneal or pelvic the extent of involvement, the child’s age at onset, and whether it is tumors) acute or chronic. Inflammatory (Crohn disease, chronic granulomatous disease) ETIOLOGY Hematoma, urinoma Ureteral obstruction occurring early in fetal life results in renal dys- Lymphocele plasia, ranging from multicystic kidney, which is associated with ure- Retroperitoneal fibrosis teral or pelvic atresia (see Fig. 537-2 in Chapter 537), to various -

Pre-Clinical Efficacy and Safety Evaluation of Human Amniotic Fluid-Derived Stem Cell Injection in a Mouse Model of Urinary Incontinence
http://dx.doi.org/10.3349/ymj.2015.56.3.648 Original Article pISSN: 0513-5796, eISSN: 1976-2437 Yonsei Med J 56(3):648-657, 2015 Pre-Clinical Efficacy and Safety Evaluation of Human Amniotic Fluid-Derived Stem Cell Injection in a Mouse Model of Urinary Incontinence Jae Young Choi,1* So Young Chun,2* Bum Soo Kim,1 Hyun Tae Kim,1 Eun Sang Yoo,1 Yun-Hee Shon,2 Jeong Ok Lim,2 Seok Joong Yun,3 Phil Hyun Song,4 Sung Kwang Chung,1 James J Yoo,2,5 and Tae Gyun Kwon1,2 1Department of Urology, School of Medicine, Kyungpook National University, Daegu; 2Joint Institute for Regenerative Medicine, Kyungpook National University Hospital, Daegu; 3Department of Urology, College of Medicine, Chungbuk National University, Cheongju; 4Department of Urology, College of Medicine, Yeungnam University, Daegu, Korea; 5Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA. Received: March 31, 2014 Purpose: Stem cell-based therapies represent new promises for the treatment of Revised: August 10, 2014 urinary incontinence. This study was performed to assess optimized cell passage Accepted: August 13, 2014 number, cell dose, therapeutic efficacy, feasibility, toxicity, and cell trafficking for Corresponding author: Dr. Tae Gyun Kwon, the first step of the pre-clinical evaluation of human amniotic fluid stem cell (hAF- Department of Urology, School of Medicine, Kyungpook National University, SC) therapy in a urinary incontinence animal model. Materials and Methods: 130 Dongdeok-ro, Jung-gu, The proper cell passage number was analyzed with hAFSCs at passages 4, 6, and Daegu 700-721, Korea. -

Urethral Diverticulum in Women: Diverse Presentations Resulting in Diagnostic Delay and Mismanagement
0022-5347/00/1642-0428/0 THEJOURNAL OF UROLOG~ Vol. 164,428-433, August 2000 Copyright 0 2000 by AMERICANUROLOCICAL ASSOCIATION, INC.@ Printed in U.S.A. URETHRAL DIVERTICULUM IN WOMEN: DIVERSE PRESENTATIONS RESULTING IN DIAGNOSTIC DELAY AND MISMANAGEMENT LAURI J. ROMANZI, ASNAT GROUTZ” AND JERRY G. BLAIVAS From the Departments of Obstetrics and Gynecology, and Urology, Weill Medical College, Cornell University, New York, New York ABSTRACT I Purpose: We describe various clinical presentations of urethral diverticulum, which may mimic other pelvic floor disorders and result in diagnostic delay. Management and outcome results are reported. Materials and Methods: We reviewed retrospectively 46 consecutive cases of urethral diver- ticulum. Patient characteristics, history, clinical evaluation, management and long-term fol- lowup are reported. Results: Mean patient age plus or minus standard deviation was 36.3 t 11.7 years. Most (83%) cases were referred as diagnostic dilemmas with symptoms present for 3 months to 27 years. Mean interval between onset of symptoms to diagnosis was 5.2 years. The most common symptoms were pain (48%of cases), urinary incontinence (35%),dyspareunia (24%)and frequen- cyhrgency (22%).The number of physicians previously consulted ranged from 3 to 20 and prior therapies included oral and/or vaginal medications, anti-incontinence surgery and psychother- apy. The diverticulum was palpable on examination in 24 patients (52%),in only 6 of whom was it possible to “milk” contents per meatus. Of these 24 palpable diverticula 2 contained malig- nancy, and 2 others contained endometriosis and stones, respectively. Diagnosis was made by voiding cystourethrography in 30 cases (65%), double balloon urethrography in 5 (11%) and transvaginal ultrasound or magnetic resonance imaging in 7 (15%).Diverticula were incidental findings during vaginal surgery in 4 cases (9%). -

Urinary Retention
Urinary Retention National Kidney and Urologic Diseases Information Clearinghouse What is urinary retention? What is the urinary tract Urinary retention is the inability to and how does it work? empty the bladder completely. Urinary The urinary tract is the body’s drainage retention can be acute or chronic. Acute system for removing urine, which is urinary retention happens suddenly and composed of wastes and extra fluid. In lasts only a short time. People with acute order for normal urination to occur, all urinary retention cannot urinate at all, body parts in the urinary tract need to work even though they have a full bladder. together in the correct order. Acute urinary retention, a potentially life-threatening medical condition, Kidneys. The kidneys are two bean-shaped requires immediate emergency treatment. organs, each about the size of a fist. They Acute urinary retention can cause great are located just below the rib cage, one discomfort or pain. on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of Chronic urinary retention can be a long- blood to produce about 1 to 2 quarts of lasting medical condition. People with urine. The kidneys work around the clock; chronic urinary retention can urinate. a person does not control what they do. However, they do not completely empty all of the urine from their bladders. Ureters. Ureters are the thin tubes of Often people are not even aware they muscle—one on each side of the bladder— have this condition until they develop that carry urine from each of the kidneys to another problem, such as urinary the bladder. -

Localization of Glucocorticoid Receptor Interacting Protein 1 in Murine
European Journal of Endocrinology (2001) 145 323±333 ISSN 0804-4643 EXPERIMENTAL STUDY Localization of glucocorticoid receptor interacting protein 1 in murine tissues using two novel polyclonal antibodies Rami Puustinen1,2, Nanna Sarvilinna1,2, Tommi Manninen1,2, Pentti Tuohimaa3 and Timo Ylikomi1,4 1Department of Cell Biology, Medical School, University of Tampere, FIN-33014, Tampere, 2Institute of Medical Technology, University of Tampere, PO Box 607, FIN-33101, Tampere, 3Department of Anatomy, Medical School, University of Tampere, FIN-33014, Tampere, and 4Department of Clinical Chemistry, Tampere University Hospital, PO Box 2000, FIN-33521, Tampere, Finland (Correspondence should be addressed to R Puustinen or N Sarvilinna; Email: rp58671@uta.® or nu56352@uta.®) Abstract Objective: Glucocorticoid receptor interacting protein 1 (GRIP1) is a coactivator that binds to the nuclear hormone receptors in a ligand-dependent manner and mediates transcriptional activation of the target genes. The aim of this study was to investigate GRIP1 expression in various murine tissues and whether the protein is nuclear, cytoplasmic, or both. Design: Two novel polyclonal antibodies against amino acids 34±47 and 468±481 of GRIP1 were raised and characterized in order to study the GRIP1 expression with immunohistochemistry. Results: Transient transfection studies with COS cells showed a clearly nuclear staining pattern and also immunohistochemical localization of GRIP1 was mainly nuclear, but cytoplasmic expression was seen as well. GRIP1 was expressed in epithelial cells of the submandibular gland, gastrointestinal tract, pancreas, kidney, uterus, mammary gland, testis, prostate, trachea, lungs and adrenal gland. GRIP1 was also detected in stromal cells of colon, rectum, urinary bladder, vagina, uterus, mammary gland and trachea, and to a lesser extent in esophagus, ureter, urethra, thymus and spleen.