Geometry of Gene Regulatory Dynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On Ramified Covers of the Projective Plane I: Segre's Theory And
ON RAMIFIED COVERS OF THE PROJECTIVE PLANE I: SEGRE’S THEORY AND CLASSIFICATION IN SMALL DEGREES (WITH AN APPENDIX BY EUGENII SHUSTIN) MICHAEL FRIEDMAN AND MAXIM LEYENSON1 Abstract. We study ramified covers of the projective plane P2. Given a smooth surface S in Pn and a generic enough projection Pn → P2, we get a cover π : S → P2, which is ramified over a plane curve B. The curve B is usually singular, but is classically known to have only cusps and nodes as singularities for a generic projection. Several questions arise: First, What is the geography of branch curves among all cuspidal-nodal curves? And second, what is the geometry of branch curves; i.e., how can one distinguish a branch curve from a non-branch curve with the same numerical invariants? For example, a plane sextic with six cusps is known to be a branch curve of a generic projection iff its six cusps lie on a conic curve, i.e., form a special 0-cycle on the plane. We start with reviewing what is known about the answers to these questions, both simple and some non-trivial results. Secondly, the classical work of Beniamino Segre gives a complete answer to the second question in the case when S is a smooth surface in P3. We give an interpretation of the work of Segre in terms of relation between Picard and Chow groups of 0-cycles on a singular plane curve B. We also review examples of small degree. In addition, the Appendix written by E. Shustin shows the existence of new Zariski pairs. -

Combination of Cubic and Quartic Plane Curve
IOSR Journal of Mathematics (IOSR-JM) e-ISSN: 2278-5728,p-ISSN: 2319-765X, Volume 6, Issue 2 (Mar. - Apr. 2013), PP 43-53 www.iosrjournals.org Combination of Cubic and Quartic Plane Curve C.Dayanithi Research Scholar, Cmj University, Megalaya Abstract The set of complex eigenvalues of unistochastic matrices of order three forms a deltoid. A cross-section of the set of unistochastic matrices of order three forms a deltoid. The set of possible traces of unitary matrices belonging to the group SU(3) forms a deltoid. The intersection of two deltoids parametrizes a family of Complex Hadamard matrices of order six. The set of all Simson lines of given triangle, form an envelope in the shape of a deltoid. This is known as the Steiner deltoid or Steiner's hypocycloid after Jakob Steiner who described the shape and symmetry of the curve in 1856. The envelope of the area bisectors of a triangle is a deltoid (in the broader sense defined above) with vertices at the midpoints of the medians. The sides of the deltoid are arcs of hyperbolas that are asymptotic to the triangle's sides. I. Introduction Various combinations of coefficients in the above equation give rise to various important families of curves as listed below. 1. Bicorn curve 2. Klein quartic 3. Bullet-nose curve 4. Lemniscate of Bernoulli 5. Cartesian oval 6. Lemniscate of Gerono 7. Cassini oval 8. Lüroth quartic 9. Deltoid curve 10. Spiric section 11. Hippopede 12. Toric section 13. Kampyle of Eudoxus 14. Trott curve II. Bicorn curve In geometry, the bicorn, also known as a cocked hat curve due to its resemblance to a bicorne, is a rational quartic curve defined by the equation It has two cusps and is symmetric about the y-axis. -
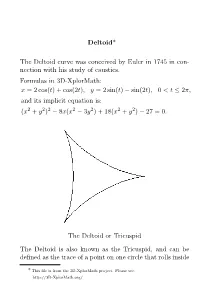
Deltoid* the Deltoid Curve Was Conceived by Euler in 1745 in Con
Deltoid* The Deltoid curve was conceived by Euler in 1745 in con- nection with his study of caustics. Formulas in 3D-XplorMath: x = 2 cos(t) + cos(2t); y = 2 sin(t) − sin(2t); 0 < t ≤ 2π; and its implicit equation is: (x2 + y2)2 − 8x(x2 − 3y2) + 18(x2 + y2) − 27 = 0: The Deltoid or Tricuspid The Deltoid is also known as the Tricuspid, and can be defined as the trace of a point on one circle that rolls inside * This file is from the 3D-XplorMath project. Please see: http://3D-XplorMath.org/ 1 2 another circle of 3 or 3=2 times as large a radius. The latter is called double generation. The figure below shows both of these methods. O is the center of the fixed circle of radius a, C the center of the rolling circle of radius a=3, and P the tracing point. OHCJ, JPT and TAOGE are colinear, where G and A are distant a=3 from O, and A is the center of the rolling circle with radius 2a=3. PHG is colinear and gives the tangent at P. Triangles TEJ, TGP, and JHP are all similar and T P=JP = 2 . Angle JCP = 3∗Angle BOJ. Let the point Q (not shown) be the intersection of JE and the circle centered on C. Points Q, P are symmetric with respect to point C. The intersection of OQ, PJ forms the center of osculating circle at P. 3 The Deltoid has numerous interesting properties. Properties Tangent Let A be the center of the curve, B be one of the cusp points,and P be any point on the curve. -

Steiner's Hat: a Constant-Area Deltoid Associated with the Ellipse
KoG•24–2020 R. Garcia, D. Reznik, H. Stachel, M. Helman: Steiner’s Hat: a Constant-Area Deltoid ... https://doi.org/10.31896/k.24.2 RONALDO GARCIA, DAN REZNIK Original scientific paper HELLMUTH STACHEL, MARK HELMAN Accepted 5. 10. 2020. Steiner's Hat: a Constant-Area Deltoid Associated with the Ellipse Steiner's Hat: a Constant-Area Deltoid Associ- Steinerova krivulja: deltoide konstantne povrˇsine ated with the Ellipse pridruˇzene elipsi ABSTRACT SAZETAKˇ The Negative Pedal Curve (NPC) of the Ellipse with re- Negativno noˇziˇsnakrivulja elipse s obzirom na neku nje- spect to a boundary point M is a 3-cusp closed-curve which zinu toˇcku M je zatvorena krivulja s tri ˇsiljka koja je afina is the affine image of the Steiner Deltoid. Over all M the slika Steinerove deltoide. Za sve toˇcke M na elipsi krivulje family has invariant area and displays an array of interesting dobivene familije imaju istu povrˇsinui niz zanimljivih svoj- properties. stava. Key words: curve, envelope, ellipse, pedal, evolute, deltoid, Poncelet, osculating, orthologic Kljuˇcnerijeˇci: krivulja, envelopa, elipsa, noˇziˇsnakrivulja, evoluta, deltoida, Poncelet, oskulacija, ortologija MSC2010: 51M04 51N20 65D18 1 Introduction Main Results: 0 0 Given an ellipse E with non-zero semi-axes a;b centered • The triangle T defined by the 3 cusps Pi has invariant at O, let M be a point in the plane. The Negative Pedal area over M, Figure 7. Curve (NPC) of E with respect to M is the envelope of • The triangle T defined by the pre-images Pi of the 3 lines passing through points P(t) on the boundary of E cusps has invariant area over M, Figure 7. -

Steiner's Hat: a Constant-Area Deltoid Associated with the Ellipse
STEINER’S HAT: A CONSTANT-AREA DELTOID ASSOCIATED WITH THE ELLIPSE RONALDO GARCIA, DAN REZNIK, HELLMUTH STACHEL, AND MARK HELMAN Abstract. The Negative Pedal Curve (NPC) of the Ellipse with respect to a boundary point M is a 3-cusp closed-curve which is the affine image of the Steiner Deltoid. Over all M the family has invariant area and displays an array of interesting properties. Keywords curve, envelope, ellipse, pedal, evolute, deltoid, Poncelet, osculat- ing, orthologic. MSC 51M04 and 51N20 and 65D18 1. Introduction Given an ellipse with non-zero semi-axes a,b centered at O, let M be a point in the plane. The NegativeE Pedal Curve (NPC) of with respect to M is the envelope of lines passing through points P (t) on the boundaryE of and perpendicular to [P (t) M] [4, pp. 349]. Well-studied cases [7, 14] includeE placing M on (i) the major− axis: the NPC is a two-cusp “fish curve” (or an asymmetric ovoid for low eccentricity of ); (ii) at O: this yielding a four-cusp NPC known as Talbot’s Curve (or a squashedE ellipse for low eccentricity), Figure 1. arXiv:2006.13166v5 [math.DS] 5 Oct 2020 Figure 1. The Negative Pedal Curve (NPC) of an ellipse with respect to a point M on the plane is the envelope of lines passing through P (t) on the boundary,E and perpendicular to P (t) M. Left: When M lies on the major axis of , the NPC is a two-cusp “fish” curve. Right: When− M is at the center of , the NPC is 4-cuspE curve with 2-self intersections known as Talbot’s Curve [12]. -

The Unexpected Hanging and Other Mathematical Diversions
AND OTHER MATHEMATICAL nIVERSIONS OLLECTION OF GI ES FRON A ERIC" The Unexpected Hanging A Partial List of Books by Martin Gardner: The Annotated "Casey at the Bat" (ed.) The Annotated Innocence of Father Brown (ed.) Hexaflexagons and Other Mathematical Diversions Logic Machines and Diagrams The Magic Numbers of Dr. Matrix Martin Gardner's Whys and Wherefores New Ambidextrous Universe The No-sided Professor Order and Surprise Puzzles from Other Worlds The Sacred Beetle and Other Essays in Science (ed.) The Second Scientific American Book of Mathematical Puzzles and Diversions Wheels, Life, and Other Mathematical Entertainments The Whys of a Philosopher Scrivener MARTIN GARDNER The Unexpected Hanging And Other Mathematical Diversions With a new Afterword and expanded Bibliography The University of Chicago Press Chicago and London Material previously published in Scientific American is copyright 0 1961, 1962, 1963 by Scientific American, Inc. All rights reserved. The University of Chicago Press, Chicago 60637 The University of Chicago Press, Ltd., London O 1969, 1991 by Martin Gardner All rights reserved. Originally published 1969. University of Chicago Press edition 1991 Printed in the United States of America Library of Congress Cataloging-in-Publication Data Gardner, Martin, 1914- The unexpected hanging and other mathematical diversions : with a new afterword and expanded bibliography 1 Martin Gardner. - University of Chicago Press ed. p. cm. Includes bibliographical references. ISBN 0-226-28256-2 (pbk.) 1. Mathematical recreations. I. Title. QA95.G33 1991 793.7'4-dc20 91-17723 CIP @ The paper used in this publication meets the minimum requirements of the American National Standard for Information Sciences-Permanence of Paper for Printed Library Materials, ANSI 239.48-1984. -

Collected Atos
Mathematical Documentation of the objects realized in the visualization program 3D-XplorMath Select the Table Of Contents (TOC) of the desired kind of objects: Table Of Contents of Planar Curves Table Of Contents of Space Curves Surface Organisation Go To Platonics Table of Contents of Conformal Maps Table Of Contents of Fractals ODEs Table Of Contents of Lattice Models Table Of Contents of Soliton Traveling Waves Shepard Tones Homepage of 3D-XPlorMath (3DXM): http://3d-xplormath.org/ Tutorial movies for using 3DXM: http://3d-xplormath.org/Movies/index.html Version November 29, 2020 The Surfaces Are Organized According To their Construction Surfaces may appear under several headings: The Catenoid is an explicitly parametrized, minimal sur- face of revolution. Go To Page 1 Curvature Properties of Surfaces Surfaces of Revolution The Unduloid, a Surface of Constant Mean Curvature Sphere, with Stereographic and Archimedes' Projections TOC of Explicitly Parametrized and Implicit Surfaces Menu of Nonorientable Surfaces in previous collection Menu of Implicit Surfaces in previous collection TOC of Spherical Surfaces (K = 1) TOC of Pseudospherical Surfaces (K = −1) TOC of Minimal Surfaces (H = 0) Ward Solitons Anand-Ward Solitons Voxel Clouds of Electron Densities of Hydrogen Go To Page 1 Planar Curves Go To Page 1 (Click the Names) Circle Ellipse Parabola Hyperbola Conic Sections Kepler Orbits, explaining 1=r-Potential Nephroid of Freeth Sine Curve Pendulum ODE Function Lissajous Plane Curve Catenary Convex Curves from Support Function Tractrix -

Mathematics, Course MATH40060 Differential Geometry September 23
University College Dublin Mathematics, Course MATH40060 (formerly called MATH4105) Differential Geometry url:- http://mathsci.ucd.ie/courses/math40060 September 23, 2009 Dr. J. Brendan Quigley comments to:- Dr J.Brendan.Quigley Department of Mathematics University College Dublin, Belfield ph. 716–2584, 716–2580; fax 716–1196 email [email protected]. prepared using LATEX running under Redhat Linux drawings prepared using gnuplot ii c jbquig-UCD September 23, 2009 Contents 0 Organization of math40060 semester1 yr 09-10 1 1 geometry and SO(3,R) 3 1.1 Euclidean three space R3 .................................... 3 1.1.1 notation and technicalities . 3 1.1.2 geometric concepts; distance, angle, volume, orientation . 4 1.1.3 Cauchy-Schwarz inequality (CSI) . 4 1.1.4 justifying our definition of distance xx(1.1) . 5 1.1.5 justifying our definition of angle, (formula(1.2)) . 6 1.1.6 justifying the definition of signed volume . 6 1.1.7 positively oriented orthonormal basis . 8 1.1.8 special orthogonal change of basis matrix . 8 1.2 projection, reflection, rotation . 9 1.2.1 line and plane,vector resolution . 9 1.2.2 Five linear mappings and their matrices . 9 1.2.3 Five linear mappings by similarity transform . 12 1.2.4 characteristic polynomial, traces, determinant . 14 1.3 Lie group SO(3;R) and Lie algebra so(3;R) .......................... 15 1.3.1 the Lie group SO(3;R) ................................. 15 1.3.2 equivalence of special orthogonal and rotation . 17 1.3.3 rotation continuously varying in time . 19 1.3.4 the Lie algebra so(3;R) ................................ -

The Topology of Subsets of Rn
APPENDIXA The Topology of Subsets of Rn In this appendix, we briefly review some notions from topology that are used throughout the book. The exposition is intended as a quick review for readers with some previous exposure to these topics. 1. Open and Closed Sets and Limit Points The natural distance function on Rn is defined such that for all a, b ∈ Rn, dist(a, b)=|a − b| = a − b, a − b. Its most important property is the triangle inequality: Proposition A.1 (TheTriangleInequality). For all a, b, c ∈ Rn, dist(a, c) ≤ dist(a, b)+dist(b, c). Proof. The Schwarz inequality (Lemma 1.12) says that |v, w| ≤ |v||w| for all v, w ∈ Rn.Thus, |v + w|2 = |v|2 +2v, w + |w|2 ≤|v|2 +2|v|·|w| + |w|2 =(|v| + |w|)2. © Springer International Publishing Switzerland 2016 345 K. Tapp, Differential Geometry of Curves and Surfaces, Undergraduate Texts in Mathematics, DOI 10.1007/978-3-319-39799-3 346 A. THE TOPOLOGY OF SUBSETS OF Rn So |v + w|≤|v| + |w|. Applying this inequality to the vectors pictured in Fig. A.1 proves the triangle inequality. Figure A.1. Proof of the triangle inequality Topology begins with precise language for discussing whether a subset of Euclidean space contains its boundary points. First, for p ∈ Rn and r>0, we denote the ball about p of radius r by B(p,r)={q ∈ Rn | dist(p, q) <r}. In other words, B(p,r) contains all points closer than a distance r from p. Definition A.2. -

The Cissoid of Diocles
Playing With Dynamic Geometry by Donald A. Cole Copyright © 2010 by Donald A. Cole All rights reserved. Cover Design: A three-dimensional image of the curve known as the Lemniscate of Bernoulli and its graph (see Chapter 15). TABLE OF CONTENTS Preface.................................................................................................................. xix Chapter 1 – Background ............................................................ 1-1 1.1 Introduction ............................................................................................................ 1-1 1.2 Equations and Graph .............................................................................................. 1-1 1.3 Analytical and Physical Properties ........................................................................ 1-4 1.3.1 Derivatives of the Curve ................................................................................. 1-4 1.3.2 Metric Properties of the Curve ........................................................................ 1-4 1.3.3 Curvature......................................................................................................... 1-6 1.3.4 Angles ............................................................................................................. 1-6 1.4 Geometric Properties ............................................................................................. 1-7 1.5 Types of Derived Curves ....................................................................................... 1-7 1.5.1 Evolute -

Bundle Block Adjustment with 3D Natural Cubic Splines
BUNDLE BLOCK ADJUSTMENT WITH 3D NATURAL CUBIC SPLINES DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Won Hee Lee, B.S., M.S. ***** The Ohio State University 2008 Dissertation Committee: Approved by Prof. Anton F. Schenk, Adviser Prof. Alper Yilmaz, Co-Adviser Adviser Prof. Ralph von Frese Graduate Program in Geodetic Science & Surveying c Copyright by Won Hee Lee 2008 ABSTRACT One of the major tasks in digital photogrammetry is to determine the orienta- tion parameters of aerial imageries correctly and quickly, which involves two primary steps of interior orientation and exterior orientation. Interior orientation defines a transformation to a 3D image coordinate system with respect to the camera’s per- spective center, while a pixel coordinate system is the reference system for a digital image, using the geometric relationship between the photo coordinate system and the instrument coordinate system. While the aerial photography provides the interior orientation parameters, the problem is reduced to determine the exterior orientation with respect to the object coordinate system. Exterior orientation establishes the position of the camera projection center in the ground coordinate system and three rotation angles of the camera axis to represent the transformation between the image and the object coordinate system. Exterior orientation parameters (EOPs) of the stereo model consisting of two aerial imageries can be obtained using relative and absolute orientation. EOPs of multiple overlapping aerial imageries can be computed using bundle block adjustment. Bundle block adjustment reduces the cost of field surveying in difficult areas and verifies the accuracy of field surveying during the process of bundle block adjustment. -

Smooth Hypocycloidal Paths with Collision-Free and Decoupled Multi-Robot Path Planning
International Journal of Advanced Robotic Systems ARTICLE SHP: Smooth Hypocycloidal Paths with Collision-free and Decoupled Multi-robot Path Planning Regular Paper Abhijeet Ravankar1*, Ankit A. Ravankar1, Yukinori Kobayashi1 and Takanori Emaru1 1 Laboratory of Robotics and Dynamics, Division of Human Mechanical Systems and Design, Hokkaido University, Sapporo, Japan *Corresponding author(s) E-mail: [email protected] Received 16 September 2015; Accepted 04 April 2016 DOI: 10.5772/63458 © 2016 Author(s). Licensee InTech. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract A novel ‵multi-robot map update‵ in case of dynamic obstacles in the map is proposed, such that robots update Generating smooth and continuous paths for robots with other robots about the positions of dynamic obstacles in the collision avoidance, which avoid sharp turns, is an impor‐ map. A timestamp feature ensures that all the robots have tant problem in the context of autonomous robot naviga‐ the most updated map. Comparison between SHP and other tion. This paper presents novel smooth hypocycloidal paths path smoothing techniques and experimental results in real (SHP) for robot motion. It is integrated with collision-free environments confirm that SHP can generate smooth paths and decoupled multi-robot path planning. An SHP diffuses for robots and avoid collision with other robots through local (i.e., moves points along segments) the points of sharp turns communication. in the global path of the map into nodes, which are used to generate smooth hypocycloidal curves that maintain a safe Keywords Path Smoothing, Robot Path Planning, Multi- clearance in relation to the obstacles.